- By Georgios Tzelepis
Microorganisms play a major role in controlling water and waste quality and every biological process is based on the action of microorganisms. Bacteria constitute an important group of microorganisms which are directly related to Environmental Engineering because of their crucial role in wastewater treatment. They are single celled prokaryotic organisms with a structurally and functionally simple form and various shapes, such as spherical, rod-shaped or spiral. One main characteristic of the bacterial cell is the lack of unit membrane system with exception the cytoplasmic membrane. The identification of bacteria is based on a number of different criteria including their morphological (shape, size), physiological and genetic characteristics. Their reproduction is based on the binary split with formation time of about 20 minutes. Bacteria are sensitive to pH changes and they survive under neutral conditions, although some of them can survive in a highly acidic environment. Regarding their survival temperature, they are divided into psychrophilic, mesophilic and thermophilic. Bacteria are very sensitive to temperature changes and they have an optimum growth temperature. (Darakas, 2016)
Bacteria have the capacity to degrade the organic substances (pollutants) and this is the reason why they are the most important group of organisms in terms of the public health engineering, since biological waste water treatment processes are based on their activities. The assimilation of pollutants is mainly achieved by the biological self-cleaning of the water thanks to microorganisms and specifically bacteria. The main three points of interest in the wastewater treatment is the microorganisms (bacteria), the included organic matter which constitutes food for microorganisms and the oxygen which is necessary for the energy and survival of microorganisms.
Get Help With Your Essay
If you need assistance with writing your essay, our professional essay writing service is here to help!
Generally, the metabolic diversity of organisms, and more specifically of bacteria, firstly depends on the energy source. Energy is important for the chemical reactions and is obtained from environmental sources. When the sources are chemicals, the species are called chemotrophs, while when the energy is derived from the light they are called phototrophic species. However, some bacteria have the ability to use both energy sources based on conditions.
Second classification is based on the carbon source. When they are organic compounds they are called chemoheterotrophs or photoheterotrophs respectively. Otherwise when inorganic compounds are used, bacteria are called chemoautotrophs or photoautotrophs.
Finally, chemotroph bacteria which metabolise organic chemicals for energy are called chemoorganotrophs. Contrariwise, those that use inorganic chemicals are called chemolithotrophs. There are two basic types of metabolism for chemoorganotrophs; fermentation, in which the metabolism of the substrate is without external oxidizing agent, and respiration, in which there is an external oxidizing agent. Both types of metabolism can convert a primary source of energy to one which can be used by the cells.
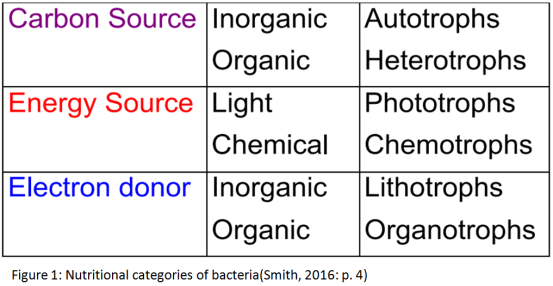
2.1.1 Carbon source
Bacteria that use carbon dioxide for the majority (or all) of their carbon requirements are called autotrophs. The obligate autotrophs that are able to use only CO2 as a source of carbon use simple energy substrates and they are either chemolithotrophs or photolithotrophs. (Singleton, 2005) In autotroph bacteria carbon dioxide from the environment is used to form complex compounds, but also there is the situation that carbon dioxide is incorporated in these compounds and called fixed. There are two common pathways for this fixation, the Calvin cycle and the reductive TCA cycle. Autotrophs are able to thrive in very harsh environments, such as deep sea vents, due to their lack of dependence on outside sources of carbon other than carbon dioxide. (Yates et al., 2016)
On the other hand, most of the known species of bacteria are heterotrophic, both aerobic and anaerobic. They use as a main source of carbon complex carbon compounds derived from other organisms, with the most significant the glucose, alcohol, and organic acids. However, there are specialised heterotrophic bacteria capable also of decomposing cellulose (actinomycetes), keratin, hydrocarbons, and other substances. Heterotrophs are only able to thrive in environments that are capable of sustaining other forms of life due to their dependence on these organisms for carbon sources. (Lester & Birkett, 1999)
2.2 Energy source
Microorganisms, and more specifically bacteria, require food to obtain energy. Phototrophic bacteria are mostly aquatic organisms and obtain energy using radiant energy (light), usually via photosynthesis. This happens through specialized pigments that they contain in order to form energy molecules. Generally, photosynthetic bacteria can be divided in two categories, these who accomplish the photosynthesis with production of oxygen (aerobically) and those without (unaerobically). (Singleton, 2005)
Chemotrophs are organisms that obtain their energy by metabolisng chemicals from the environment, through the oxidation of inorganic molecules, such as iron and magnesium. They are divided in two different categories, chemoautotrophs and chemoheterotrophs, with their difference already been described. (Boundless, 2016)
Carbon source of heterotrophic bacteria can be either soluble and colloidal organics of untreated waste (BOD) or endogenous carbon microorganisms, i.e. the carbon putrescent dead cells or methanol (CH3OH), which is the best organic substrate to the denitrification. (Darakas, 2016)
2.3 Electron acceptor
As mentioned, all the bacterial cells have to convert a primary source of energy into forms that can be used. Some cells can convert a primary energy source to an electrochemical form which consists of a gradient of ions between the two surfaces of cytoplasmic membrane. Chemotroph and phototroph bacteria form high-energy compounds from a primary energy source using different techniques. (Singleton, 2005)
Respiration is a type of metabolism in which a substrate is metabolized with the help of an external oxidizing agent. Oxygen can work as the exogenous oxidizing agent having aerobic respiration, or organic oxidizing agents can be used instead in an anaerobic respiration. Despite the fact that the oxidizing agent can be inorganic or organic, in chemoorganotrophs, the substrate is always an organic compound. (Singleton, 2005)
Oxygen is the final electron acceptor for the aerobic respiration. The sugar is completely broken down to carbon dioxide and water, yielding a maximum of 38 molecules of ATP per molecule of glucose. Electrons are transferred to oxygen using the electron transport chain (ETC), a system of enzymes and cofactors located in the cytoplasmic membrane and arranged so that the passage of electrons down the chain is coupled with the movement of protons (hydrogen ions) across the membrane and out of the cell. ETC induces the movement of positively charged hydrogen ions to the outside of the cell and negatively charged ions to its interior. This ion gradient results in the acidification of the external medium and an energized plasma membrane with an electrical charge of 150 to 200 millivolts. The generation of ion gradients is a common aspect of energy generation and storage in all living organisms. The gradient of protons is used directly by the cell for many processes, including the active transport of nutrients and the rotation of flagella. The protons also can move from the exterior of the cell into the cytoplasm by passing through a membrane enzyme called the F1F0-proton-translocating ATPase, which couples this proton movement to ATP synthesis. (Kadner & Rogers, 2015)
Bacteria that are able to use respiration produce far more energy per sugar molecule than do fermentative cells, because the complete oxidation of the energy source allows complete extraction of all of the energy available. (Kadner & Rogers, 2015)
Respiration can also occur under anaerobic conditions. Anaerobic respiration uses external oxidizing agents such as nitrate (NO3), nitrite (NO2), sulfate (SO42), or fumarate in place of oxygen. Depending on the different types or conditions, the electron donor (substrate) used by chemoorganotrophs in anaerobic respiration is of various organic compounds. The energy yields available to the cell using these acceptors are lower than in respiration with oxygen, but they are still substantially higher than the energy yields available from fermentation. The utilization of CO2as a terminal electron acceptor is limited to a group of bacteria called methanogens and this process requires a strongly reduced environment. This procedure produces methane (CH4) which can be a problem in some instances like landfill sites. (Maier, 1999)
All the bacteria have an ”optimum growth temperature” where their growth is faster, while they also have a specific range of temperature into which they can only grow. Most of the bacteria are mesophilic and they grow in temperatures between 15 and 45 degrees of Celsius. Thermophilic are bacteria with growth temperature over 45 degrees of Celsius, while psychrophilic are the bacteria with growth temperature under 15 degrees.
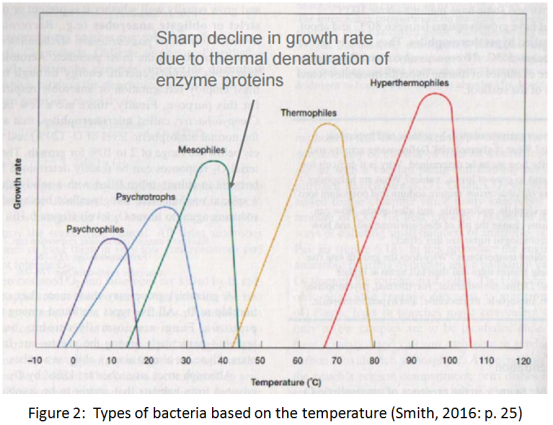
3.1 Low temperature
It is well known that bacteria as well as various other forms of life survive and thrive optimally in moderate conditions of temperature, pressure, pH and other environmental parameters. However, there is also evidence of bacteria life in extreme environments. For example bacteria were found to exist in the very acidic river Rio Tino while also bacteria were detected in subzero environments like in Lake Vostok even in depth of 3600 meters, below the surface ice. (Chattopadhyay & Sengupta, 2013)
Find Out How UKEssays.com Can Help You!
Our academic experts are ready and waiting to assist with any writing project you may have. From simple essay plans, through to full dissertations, you can guarantee we have a service perfectly matched to your needs.
View our academic writing services
At low temperature, bacteria are challenged with a number of difficulties due to decrease in the rate of biochemical reactions that sustain the life. Bacteria taken from low temperature environments were found with increased branched chain, short chain, anteiso and unsaturated fatty acids. They were also found to synthesize more cis fatty acids in preference to trans fatty acids. All these factors are contributing in the increase of membrane fluidity. Moreover, in order to adjust with the low enthalpy and the reduced atomic and molecular motions at low temperature, they achieve flexibility through reduction in strength and number of non-covalent interactions. Finally a high level of post-transcriptional modification of t-RNA by dihydrouridine also has a major role in psychrophiles. Dihydrouridine unsettles the stacking that stabilizes the RNA. (Chattopadhyay & Sengupta, 2013)
3.2 High temperature
Thermophilic bacteria are common in soil and volcanic habitats and have a limited species configuration. Examination of metabolic pathways and regulatory mechanisms in thermophiles proves that thermophilic bacteria have almost the same properties commonly found in mesophilic bacteria, with the main difference being specific molecular mechanisms, important in high temperature biological stability and activity. As a consequence of growth at high temperature and unique macromolecular properties, thermophilic bacteria can possess high metabolic rates, physically and chemically stable enzymes than similar mesophilic species. Thermophilic processes appear more stable, rapid and facilitate reactant activity and product recovery. Analysis of important biomolecules in thermophilic bacteria has revealed subtle structural differences in proteins, nucleic acids and lipids. Some of these differences have not been observed in mesophilic bacteria. For instance the membrane lipids of extreme thermophiles contain more saturated and straight chain fatty acids than mesophiles. This allows thermophilic bacteria to grow at higher temperatures by providing the correct degree of fluidity required for membrane function. Finally the explanation for high temperature stability of tRNA in Thermus species is that Thermus transfer RNA contains more guanine plus cytosine bases in the specific base-paired region, which provides greater hydrogen bonding and increased thermal stability. Also, the base-paired region in tRNAs from Thermus contains more thiolated thymidine which provides a stronger stacking force inside the molecule. (Zeikus, 1979)
The restoration, maintenance and protection of the environment with the help of biological agents in general and bacteria more specifically are significantly important in terms of sustainability in the environment. Hence, in many cases, bacteria and environmental engineering go hand in hand and both are interdependent on each other. Their main connection is the removal and treatment of the wastes, solid or liquid, from various sources like the industrial, domestic and other. There are many examples of the use of bacteria especially in waste and wastewater treatment, where some useful characteristics of bacteria are used.
4.1 Wastewater treatment
Biological treatment is one of the most widely used removal methods as well as for partial or complete stabilization of biologically degradable substances in wastewaters. General characteristics of wastewaters are measured in terms of Chemical Oxygen Demand (COD), Biochemical Oxygen Demand (BOD), and Volatile Suspended Solids (VSS). Bacteria provide the largest component of the microbial community in all biological wastewater treatment processes, and numbers in excess of 106 bacteria/ml of wastewater are frequently encountered.
4.1.1 Activation Sludge
Activated sludge is a process that has been adopted worldwide as a secondary biological treatment for domestic wastewaters. In the activated sludge process the incoming wastewater is mixed and aerated with existing biological sludge (microorganisms). Organics in the wastewater come into contact with the microorganisms and are utilized as food and oxidized to CO2, and H2O. The microorganisms using the organics as food they reproduce, grow, and die. While the microorganisms grow, are mixed together by the movement of air so individual organisms join an active mass of microbes called activated sludge. The wastewater flows continuously into an aeration tank where air is injected to mix the activated sludge with the wastewater and to supply oxygen needed for microbes to breakdown the organic materials. This mixture of activated sludge and wastewater in the aeration tank is called mixed liquor suspended solids and mixed liquor volatile suspended solids. The mixed liquor is sent to the sludge handling disposal (second part of activation sludge method). A part of this mass precipitates while the rest flows back to the aeration tank in order to maintain sufficient microbial population levels. This is the called activated sludge. The microorganisms in activated sludge generally are composed of 70 to 90% organic and 10 to 30% inorganic matter. The microorganisms generally found in activated sludge consist of bacteria (mostly), fungi and protozoa.
4.1.2 Nitrogen and Phosphorus removal
Nitrogen and phosphorus are two essential elements in terms of the waste treatment. The nitrogen compounds and the phosphates existing in wastewaters are very important for the survival of the bacteria although they should be removed in order to avoid problems of deoxygenation and eutrophication in the final recipient. (Bitton, 2010)
Nitrification
The principal organisms involved in nitrification processes belong into two categories, Nitrosomonas and Nitrobacter. These bacteria are considered to be strictly autotrophs since they derive energy for growth and synthesis from the oxidation of inorganic nitrogen and carbon (CO2) compounds. Nitrosomonas catalyse oxidation of ammonia to nitrite using molecular oxygen, while Nictobacter further oxidize nitrite to nitrate using oxygen derived from the water molecule. It should be mentioned that some some soluble forms of c-BOD can inhibit the activity of nitrifying bacteria since they are able to enter the cells of nitrifying bacteria and inactivate their enzyme systems. (Horan, 1989)
Denitrification
Denitrification is a process by which certain species of bacteria under anoxic conditions reduce nitrate nitrogen to the gaseous end-products of N2, NO, or N2O which can then escape from solution to the atmosphere. Unlike other nitrogen compounds, the gaseous forms of nitrogen have no significant effect on environmental quality. The presence of oxidized nitrogen and organic carbon are essential properties for denitrification to proceed. Denitrifying bacteria are composed of heterotrophic organisms. The most common denitrifying bacteria are Bacillus denitrijicans, Micrococcus denitrijicans and more. (Horan, 1989)
Phosphorus removal
The anaerobic-oxic process (most commonly used), consists of a modified activated sludge system that includes an anaerobic upstream of the conventional aeration tank. During the anaerobic phase, inorganic phosphorus is released from the cells as a result of polyphosphate hydrolysis. The energy liberated is used for the uptake of BOD from wastewater. (Bitton, 2010)Removal efficiency is high when the BOD/phosphorus ratio exceeds 10. During the aerobic phase, soluble phosphorus is taken up by bacteria that synthesize polyphosphates using the energy released from BOD oxidation. The anaerobic-oxic process results in BOD removal and produces sludge which is rich in phosphorus. The key features of this process are the relatively low solid retention time and high organic loading rates. (Cheremisinoff, 1997)
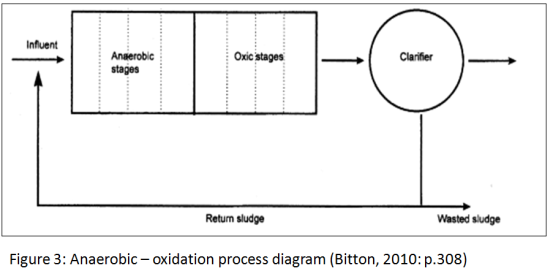
4.1.3 Anaerobic Digestion
Major applications of anaerobic digestion are the stabilization of concentrated sludges produced from the treatment of especially industrial wastes. The digestion is a complex biochemical process in which several groups of anaerobic and facultative organisms again simultaneously absorb and break down organic matter and can be described as a two-phase process. In the first phase, acid-forming organisms convert the complex organic substrate to simple organic acids. Little change occurs in the total amount of organic material in the system, with decrease in ph . Second phase involves conversion of the organic acids to principally methane and carbon dioxide. The anaerobic process is essentially controlled by the methane producing bacteria. Methane formers are very sensitive to pH, substrate composition, and temperature. If the pH drops below 6,methane formation stops, and there is no decrease in organic content of the sludge. One characteristic of the methane bacteria is that they are highly active in the mesophilic and thermophilic ranges. (Cheremisinoff, 1997)
4.2 Solid Waste Treatment
4.2.1 Composting
Composting is the biological decomposition and stabilization of organic substrates. Each gram of decaying compost contains millions of beneficial microorganisms that work to break down organic matter. Of the microorganisms present, 80 to 90 percent are bacteria, including actinomycetes and aerobic bacteria. Aerobic are separated in three different varieties, each of which is active at different phases of the decomposition process. Psychrophilic bacteria (during winter) work on the initial organic matter, at temperatures around 12 degrees Celsius. These bacteria raise the temperature to 20 C, at which time, the mesophilic bacteria take over. These bacteria work at moderate to warm temperatures between 20 and 38 C. At 38 C, the thermophilic bacteria take over, raising the temperature to 70 C. Once this happens, the process starts over again with the addition of new materials. Actinomycete bacteria appear during the late stages of composting to clean up remaining materials that are difficult for aerobic bacteria to break down. They are responsible for breaking down cellulose, proteins, lignin and starches.
References
- Bitton G., (2010), Activated Sludge Process, in Wastewater Microbiology, 4th Edition, Hoboken, NJ, USA, John Wiley & Sons, Inc.
- Boundless, (2016), Chemoautotrophs and Chemoheterotrophs, Boundless Microbiology, Available from: https://www.boundless.com/microbiology/textbooks/boundless-microbiology-textbook/microbial-metabolism-5/types-of-metabolism-41/chemoautotrophs-and-chemoheterotrophs-285-6153/, [Accessed: 13 January 2017]
- Chattopadhyay M. and Sengupta D., (2013), Metabolism in bacteria at low temperature: A recent study report., Biosciences, 31, 2, 157-165. Available from: https://www.researchgate.net/publication/236674848_Metabolism_in_bacteria_at_low_temperature_A_recent_report , [Accessed: 10 January 2017]
- Cheremisinoff N. P., (1997), Biotechnology for Industrial and municipal wastes, in Biotechnology for Waste and Wastewater Treatment, 1-36
- Darakas E., (2016), Environmental Engineering: Process of water and wastewater treatment, Thessaloniki, Sofia Publisher.
- Horan N., (1989), Biological Wastewater Treatment Systems: Theory and Operation., Chichester, England, Wiley – Blackwell.
- Hurst, C.J. et al., (2002), Manual of Environmental Microbiology, 2nd Edition, Washington, ASM Press.
- Kadner R. J. and Rogers K., (2015), Bacteria. Available from: https://www.britannica.com/science/bacteria/Salt-and-water. [Accessed: 23 December 2017]
- Lester, J.N. & Birkett, J.W., (1999), Microbiology and Chemistry for Environmental Engineers, London, E. & F.N. Spon.
- Maier, E.M. et al, (1999), Environnemental Microbiology, Academic Press
- Singleton P., (2005), Bacteria in Biology, Biotechnology and Medicine, 6th Edition, Wiley
- Smith S. R., (2016), Bacteria, Lecture Slides for the course of Microbiology for Environmental Engineering; MSc of Environmental Engineering, London, Imperial College London.
- Traumann N. and Olynciw E., (1996), Compost Microorganisms, Cornell Waste Management Institue, New York, Available from: http://compost.css.cornell.edu/microorg.html, [Accessed: 10 January 2017].
- Yates V. M., Nakatsu C. H., Miller R. V., Pillai S. D., (2016), Manual of Environmental Microbiology, 4th Edition, ASM Press
- Zeikus, J.G., (1979), Thermophilic bacteria: ecology, physiology and technology., Enzyme and Microbial Technology, 1, 4, 243-252. Available from: http://www.sciencedirect.com/science/article/pii/0141022979900437?via%3Dihub, [Accessed: 27 December 2016]
Cite This Work
To export a reference to this article please select a referencing style below:


