CdS Quantum dots: Synthesis and Optical Properties Characterization for Solar Cell
- Raimy Roy
Abstract— In this work CdS quantum dots were synthesized using Successive Ionic Layer Adsorption and Reaction (SILAR) method. Then a study of the morphology and optical property were made for the application of solar cell. The structural characterization were made by XRD while the optical characterization where done by UV-vis-NIR spectroscopy techniques.
Index Terms—Quantum dots, SILAR
I. INTRODUCTION
Quantum dot sensitized solar cell is an emerging field of photovoltaic in which the absorbing material is a quantum dot. The advantage of using such solar cell is size tunability and increased surface to volume ratio.
In a quantum dot based solar cell the active layer consist of the quantum dot and the scattering layer is formed by the TiO2 layer. The mesoscopic TiO2 when deposited with CdS quantum dot act as an energy harvester and convert the incident photon to electricity. In this work, a model of the photoanode for the solar cell was made with mesoscopic TiO2 layer as scattering layer and quantum dots as absorbing layer. Here instead of ITO a glass slide was used. [1]
Get Help With Your Essay
If you need assistance with writing your essay, our professional essay writing service is here to help!
To synthesize a quantum dot various techniques are used. Among them Successive Ionic Adsorption and Reaction (SILAR) method is a cost effective and is used to prepare quantum dot. In a SILAR method the time of reaction or the number of cycles can be controlled. Depending on which the size of the quantum dot varies. Another advantage of this technique is that it can be prepared at room temperature. Also this method provides a close contact between the quantum dots and the oxide layer, so it is an attractive method for the preparation of electrodes in a solar cell. [1]
Cadmium sulfide (CdS) quantum dot is a direct band gap semiconductor. It is a II-VI compound semiconductor that is used for many optoelectronic devices such as solar cell, laser diodes and photoconductors. It is an inorganic semiconductor which has several advantages over conventional dyes. These advantages are band gap tunability, large extinction coefficient (this means that the dark current can be reduced and the overall efficiency can be improved) and multiple electron generation by utilizing hot electrons. [2]
II. EXPERIMENTAL SETUP
- Chemicals Required
Titanium dioxide powder (SD Fine-Chem Limited, purity 60%), 2M nitric acid, 0.05M cadmium nitrate, ethanol, 0.05M sodium sulfide hydrate (Sigma Aldrich, assay=60%), methanol.
- Preparation of TiO2 layer on glass slide
A paste of titania (TiO2) was prepared from TiO2 powder and nitric acid. The chemicals were added in 2:1 proportion. A thin layer of titania paste was coated on the glass slide using a technique called doctor blade method [3]. In this method, either a glass rod or a microscope slide is used. We have used a microscope slide of thickness 1.45mm to coat the paste. A glass slide of dimension 2cm X 1cm was cut and cleaned. With the help of an adhesive tape, the glass slide is positioned firmly on the work bench. Another advantage of using such tape is that we could define an area to coat the paste and to deposit the quantum dot. Now place the paste on one side of the glass slide, positioning the microscope slide in 45ï‚° spread the paste across the glass slide. Repeat the operation till a reasonably homogeneous layer is formed. After coating heat the paste to 80ï‚°C followed by annealing at 450ï‚°C for 30 min. After sintering the paste is white in color. This provides a better surface for adsorption of the CdS quantum dots since sintering makes the mesoporous films to a continuous network.
- Deposition of CdS Quantum Dots
Successive Ionic-Adsorption and Reaction method is commonly used to deposit metal sulphide onto a nanostructured film. CdS quantum dot was deposited onto titania using this method as described in [4]. The first precursor solution used is 0.05M cadmium nitrate (Cd(NO3)2) and the second precursor solution is 0.05M sodium sulphide (Na2S). The bare TiO2 paste is dipped onto the first precursor solution for one minute. The Cd2+ ions have been deposited onto the TiO2 surface. This is then rinsed in an ethanolic solution for one minute and dried under room temperature. It is then dipped in the anionic precursor for one minute and then rinsed in methanolic solution for one minute and allowed to dry at room temperature. This completes one deposition cycle of SILAR. In this work we have performed four deposition cycles of SILAR.
III. RESULT AND DISCUSSION
The CdS quantum dot was deposited on to the surface of TiO2. An obvious color change was observed during the deposition cycle which is shown in Fig.1. The color change was pale yellow to golden yellow. The characterization was done using XRD and UV-vis spectroscopy techniques.

Fig 1: Photograph of glass slides with CdS coating with increasing SILAR cycles
- XRD Characterization
Fig 2. shows the obtained XRD pattern for TiO2 (Fig.2a), TiO2/ CdS (Fig 2b.) . From the peak obtained, we confirm that CdS quantum dot was deposited onto the film. Since the peaks at 44.1ï‚°, 51.9ï‚°, 64.3ï‚°, 70.4ï‚° and 72.9ï‚° coincides with the intensity pattern as defined by the JCPDS 10-0454 for the CdS QD. The corresponding miller indices are (220), (311), (400), (331) and (420). From this we conclude that CdS QD was deposited. It belongs to the cubic crystal system and the mineral name is hawleyite. For TiO2 the XRD pattern exactly matches with JCPDS 21-1272. It belongs to tetragonal crystal system and its mineral name is anatase.
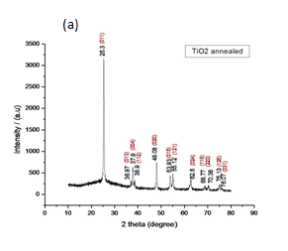

Fig.2 : XRD pattern (a) TiO2 (b) TiO2/CdS
- Size Characterization
The size characterization was done by non-contact mode AFM (Atomic Force Microscopy). The size of the CdS quantum dot was found to be 25.83nm. the thickness of the deposited layer was calculated to be 29.65nm. Fig 3.

Fig 3 : AFM non-contact mode characterization of CdS quantum dot
- UV-vis Characterization
The optical property was characterized using Jasco Spectrophotometer V670. The absorption spectrum is shown in Fig 4. The absorption spectrum for the TiO2 and CdS/TiO2 is shown in Fig 4a. and TiO2/CdS alone is shown in Fig.4b. The absorption peak for CdS is as reported by Antonio et.al [4]. From the absorption spectrum we could observe a shift in the peak indicating CdS QD is being deposited. The absorption peak was observed in the range of 386nm-484nm. For TiO2 the absorption peak was observed at 341nm. In Fig 4b. the inset is the absorption spectrum that was reported in [5]

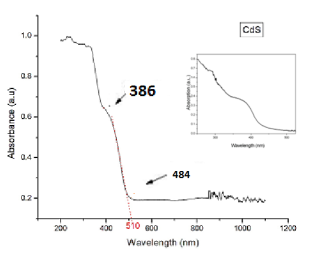
Fig 4: Absorption spectrum of (a) TiO2 and TiO2/CdS (b) TiO2/CdS

Fig 5: UV-Vis absorption spectra showing increase (~49 %) in absorption due to CdS
Figure 5. depicts the percentage increase in the absorption peak of CdS with respect to TiO2. It was calculated to be a 49.08% increase in the absorption peak.
- Determination of Optical Band gap
The DRS (Diffuse Reflectance Spectroscopy) characterization was done to obtain the optical band gap. The optical band gap was calculated by plotting the Tauc plot . It is the plot between energy and absorbance. The optical band gap can be determined by Tauc relation

Where ï¡ is the absorption coefficient in cm-1, hï® is the photon energy in eV and A is a constant. The value of n is given as follows
n = ½ for direct allowed transition
n = 2 for indirect allowed transition
The Tauc plot for TiO2 and TiO2/CdS is shown in Fig 6. TiO2 is an indirect band gap material whereas CdS is a direct band gap semiconductor. The bandgap value of CdS in bulk is given as 2.42eV [5]. From the experiment we calculated the optical band gap to be 2.38eV. Also the absorbance value of the CdS QD is blue shifted. Using the equation
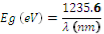
The value of the peak was calculated to be 519.16nm which is within the absorption region.

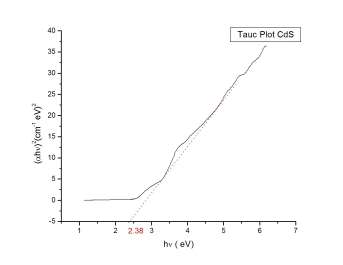
Fig 6. Tauc plot of (a) TiO2 (b)TiO2/CdS
IV. CONCLUSION
In this work CdS quantum dot have been synthesized using SILAR method. Its structural characterization was done that confirmed the deposition of the CdS quantum dot on to TiO2 paste. The optical property was characterized and analysed using UV-vis-NIR spectroscopy. The optical band gap was calculated to be 2.38 eV. The size of the quantum dot deposited was calculated to be in nanometer.
REFERENCES
[1] Prashant V Kamat , “Quantum dot Solar cells.The next Big Thing in Photovoltaics” J.Phys.Chem.Lett. 2013, 4, 908-918.
[2] Chang Liu,Yitan Li,Lin Wei,Cuncun Wu,Yanxue Chen,Liangmo MeiandJun Jiao, “CdS quantum dot-sensitized solar cells based on nano-branched TiO2arrays” Nanoscale Research Letters 2014,9.
[3] A. Berni, M. Mennig, H. Schmidt, “Doctor blade method”, Springer.
[4] Antonio Braga,SixtoGimenez, Isabella Concina, Alberto Vomiero and Ivan Mora-Ser, “Panchromatic Sensitized Solar Cells Based on Metal Sulfide Quantum Dots Grown Directly on Nanostructured TiO2 Electrodes”, J. Phys. Chem. Lett. 2011, 2, 454–460.
[5] B. T. Huy, Min-Ho Seo, Jae-Min Lim, Dong-Soo Shin and Yong-Ill Lee, “A Systematic Study on Preparing CdS Quantum Dots” Journal of the Korean Physical Society, Vol. 59, No. 5, November 2011, 3293-3299
Cite This Work
To export a reference to this article please select a referencing style below:


