Introduction
The Direct Hydrocarbon Fuel cell or Solid Oxide Fuel cells (SOFC), as its name suggests contains a solid oxide material as an electrolyte that has a unique ability to conduct an oxygen electrolyte. This oxygen ion from cathode travels to the anode side where it reacts with the hydrocarbon fuel to generate electricity. Due to this ability, the SOFC can theoretically oxidize any Hydrocarbon Fuel to produce electricity. Also, in a conventional Proton exchange membrane fuel cell (PEMFC) which uses Hydrogen gas to produce electricity, most of the hydrogen comes from refining from Hydrocarbon sources such as Methane. In the present state, nearly 96% of Hydrogen is produced by such process [4]and nearly 20-30% of the fuel value is lost in the conversion process [4]. Another limitation of the PEMFC is that the storage and transport of high-density Hydrogen are difficult and expensive. The major advantage of SOFC is that since the Oxygen anion is being transported through the membrane, these fuel cells can practically work on any combustible hydrocarbon fuel. Moreover, due to the high operating temperature, the exhaust heat can be utilized to produce steam and produce power using a combined cycle. For Standalone applications, the SOFC provides an efficiency of 45-65% [13] which is twice that of an Internal Combustion Engine. I have compiled the working, problems, and improvements in Cathode materials of SOFC in this literature review paper.
Get Help With Your Essay
If you need assistance with writing your essay, our professional essay writing service is here to help!
Cathode-Kinetics and Reaction mechanism, Materials
Oxygen is the single diatomic species involved in the overall electrolytic reactions at the cathode. The steps that occur when O2 interacts with cathode are (1) The oxygen gets adsorbed and reduced to oxygen anion and the oxygen ion gets incorporated into the lattice of the porous cathode. (2) the oxygen ion gets transferred to the electrolyte from the porous cathode. (3) ion is transported to the electrolyte lattice. The reaction is as follows, ½ O2 (gas) + 2e- (Cathode) O2-

Figure 1: Oxygen-Cathode interactions
A composite of Sr doped LaMnO3 is generally preferred for the use of high-temperature SOFC from 800-1000°C. Since the SOFC operates at high temperatures, the cathode materials must remain stable in the operating range and also must maintain the porosity. Due to this, the choice of materials limits to noble metals or solid oxides. Due to economic reasons, solid oxides are generally used as cathode materials.
Lanthanum Magnetite
One of the widely used materials for cathode materials is the Strontium-doped Lanthanum magnetite (LSM) and the general formula is La1-xSrxMnO3. The conductivity of lanthanum magnetite is basically of p-type and at high temperatures, its oxygen stoichiometry is a function of partial pressure of oxygen in the oxidizing environment. The p-type conductivity is due to the formation of vacancies at either A or B sites. The conductivity can be improved by adding Alkaline earth metal dopants such as Magnesium, calcium, strontium or barium. Strontium has the most excellent electrical properties and is, therefore, strontium doped lanthanum magnetite is widely used. One problem in the manufacturing of LSM is that it starts to react with the zirconia electrolyte at 1300°C. Therefore, limiting its sintering temperature.
Manganite based materials generally have very low conductivities at temperatures below 800°C. Mostly the manganite based compounds are formed by strontium doping at the A-site, However, Calcium doped materials such as Pr0.7Ca0.3MnO3 have proved to be more promising and are also thermally and chemically stable. Scandium doped manganite La0.8Sr0.2Mn1-xScxO3 (LSMS) further reduces the operating temperature. Moreover, this is due to the nonstoichiometric defects that occur due to Sc substitution that further increases the mobility of oxygen ion. However, high doping (>10% / mol) causes segregation of secondary phases resulting in a dip in performance.

Figure 2: Comparison of conductivity of perovskite materials
Lanthanum Cobalt Oxide
Lanthanum cobalt oxide has much better electrical conductivity as compared to Lanthanum magnetite under the same working conditions. There are also many drawbacks in using the LaCoO3. The LaCoO3 has greater reactivity towards the zirconia electrolyte at higher temperatures and also it is more prone to self-reduction at high temperatures. Moreover, the thermal expansion coefficient of LaCoO3 is much higher than the zirconia electrolyte. This possesses additional problem in durability and interconnecting the cathode with the electrolyte. LaCoO3 is mostly mixed with LSM to obtain better electrical and thermal properties.
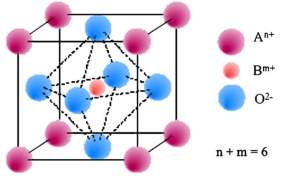
Figure 3: Typical structure of Perovskite materials (ABO3)
The ionic conductivity can be further enhanced the ionic conductivity and catalytic activity of (La, Sr) CoO3. Pr+3, when used as rare earth cations at A-site, exhibits the highest electrical conductivity and lowest overpotential values due to valence change from +3 to +4.
Another widely used cobalt-based cathode material is Sr-doped samarium cobaltite (Sm1-xSrxCoO3) as these also operate at intermediate temperature ranges (500-600°C). The maximum conductivity is observed at x=0.5 and the conductivity peaks at 103 S/cm at higher temperatures.
Along with the reactivity of cobalt-based compounds with zirconia electrolyte at higher temperatures during their sintering reduces their performance. To avoid this, a diffusion barrier layer is necessary between the Cobalt-based cathode and zirconia electrolyte.
A perovskite material for anode has a general form of ABO3. Here A and B are cations having a total charge of +6. The A cations are La, Sr, Ca and Pb. B cations are Tim Cr, Ni, Co, Fe, and Zr.
Barium Strontium Cobalt Ferrite (BACF)
BACF is a new cathode material that has high-performance characteristics. This material also operates at a much lower temperature (500-700°C). The traditional materials have very low oxygen electrochemical reduction properties at this temperature. BACF is generally constructed in thin films doped with ceria. It exhibits a high-power density of 1010 mW cm-2 at 500-600 degrees C. BACF has excellent performance due to high oxygen diffusion through the material. As studied by Zhou et al, the performance of BACF can further be improved by adding silver as it helps in oxygen ion mobility.
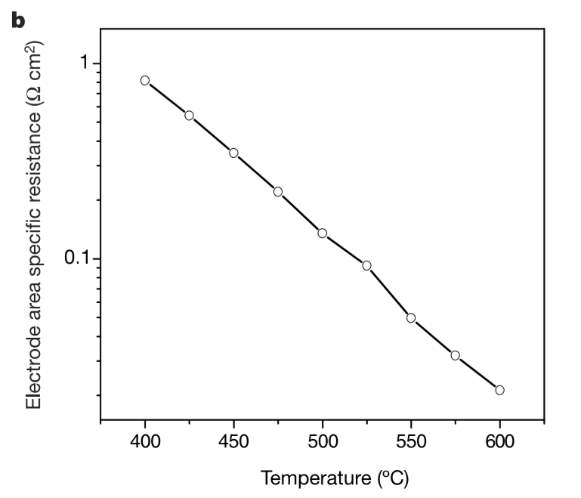
Figure 4: Variation of specific resistance with temperature for BACF
Lanthanum Ferrite Cathodes
Lanthanum Ferrite (LaFeO3) are more stable compared to cobalt-based perovskites because Fe3+ has a stable electronic composition of 3d5. They have a stable performance with respect to power density and stability at 750 degrees C. Moreover, in these iron-based electrodes, the reactivity with the YSZ electrolyte is significantly reduced. The total conductivity in these electrodes is dominated by the hole conduction mechanism. Moreover, these materials do not show any signs of degradation over a 500h test that indicates a better material for SOFC. Also, from figure-4, it is evident that increasing the concentration reduces the thermal expansion coefficient of the electrode materials. This can be helpful in manufacturing Cobalt-Iron based composites that have higher electrical conductivity and lower thermal expansion coefficient (TEC) implying greater performance and durability. The optimum Co: Fe was experimentally found out to be 0.7:0.3 Moreover, adding strontium to lanthanum ferrites further decreases the resistivity. It was found that the resistivity of La0.8Sr0.2FeO3 was several magnitudes lower than LaFeO3. However, the introduction of aluminum increased the resistivity of the material. Also, the resistivity of these composites was found to be temperature independent after 673K. This makes them good materials for Low-Temperature SOFC.
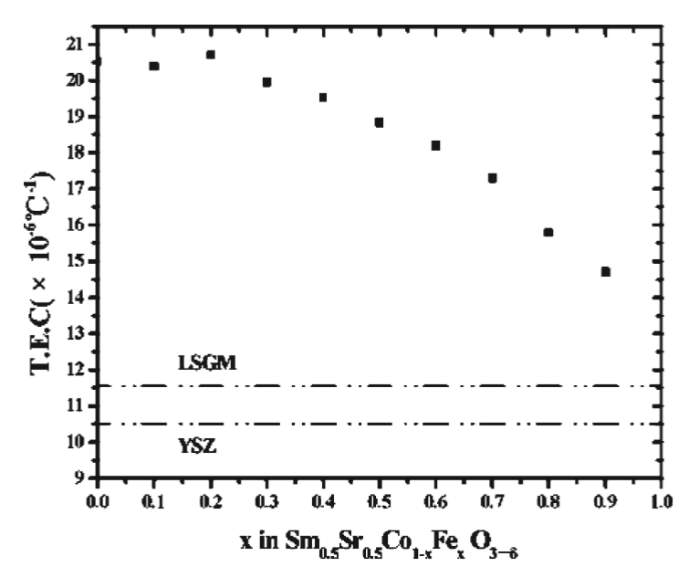
Figure 5: Variation in thermal expansion coefficient (TEC) with increasing iron content.
Conclusion
Solid Oxide Fuel Cells provide a unique blend of operating on traditional hydrocarbon fuels and directly producing electricity with very high efficiency. These characteristics move them to the top right corner of the Ragone plot. There are many limitations with the widely used LSM cathodes as they cannot operate below 800°C. There is currently much interest in developing materials that operate as low as 500°C and some promise is being shown by the composite cathodes.
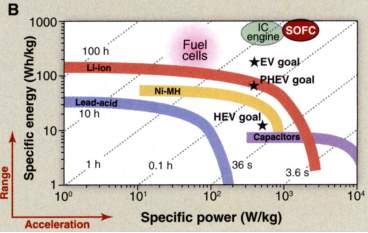
Figure 6: Ragone Plot including IC engine and SOFC.
References
Solid oxide fuel cells- R. Mark Ormerod. Birchall Centre for Inorganic Chemistry and Materials Science, School of Chemistry and Physics, Keele University, Staffordshire, UK ST5 5BG.
A high-performance cathode for the next generation of solid-oxide fuel cellsZongping Shao & Sossina M. Haile Materials Science, California Institute of Technology, Pasadena.
Cathode materials for solid oxide fuel cells: a review Chunwen Sun & Rob Hui & Justin Roller J Solid State Electrochem (2010) 14:1125–1144 DOI 10.1007/s10008-009-0932-0
Direct Hydrocarbon Solid Oxide Fuel Cells Steven McIntosh and Raymond J. Gorte* Chem. Rev. 2004, 104, 4845−4865
Electrode materials and reaction mechanisms in solid oxide fuel cells: a brief review. J Solid State Electrochem (2008) 12:1367–1391 DOI 10.1007/s10008-008-0611-6
Intermediate temperature solid oxide fuel cells. Advance Article on the web 28th May 2008 DOI: 10.1039/b612060c
Materials and manufacturing technologies for solid oxide fuel cells. J Mater Sci (2010) 45:3109–3135 DOI 10.1007/s10853-010-4279-9
Materials for Solid Oxide Fuel Cells. Department of Chemistry, University of Houston, Houston, Texas, 77204-5003 Chem. Mater. 2010, 22, 660–674 DOI:10.1021/cm902640j.
Recent advances in Perovskite-type materials for solid oxide fuel cell cathodes. Centre for Ion Conducting Membranes, Department of Materials, Imperial College of Science ,Technology and Medicine.
Solid Oxide Electrolysis Cells: Degradation at High Current Densities. The Electrochemical Society. DOI: 10.1149/1.3447752.
Application of solid oxide fuel cell technology for power generation—A review. Renewable and Sustainable Energy Reviews 20(2013)430–442.
Factors Governing Oxygen Reduction in Solid Oxide Fuel Cell Cathodes. Chem. Rev. 2004, 104, 4791−4843
Lowering the Temperature of Solid Oxide Fuel Cells Science, New Series, Vol. 334, No. 6058 (pp. 935-939) Published by: American Association for the Advancement of Science.
Cite This Work
To export a reference to this article please select a referencing style below:


