Effect of synthesis temperature on the morphology and pseudocapacitive behavior of Nickel-Cobalt layered double hydroxides
Li-Ming Lua, Shan-Shan Xua, Rui-Qi Fenga, Zhan-Bing He b, Tie-Zhen Rena* and Teresa J. Bandoszc
a School of Chemical Engineering, Hebei University of Technology, Tianjin 300130 China
b State Key Laboratory for Advanced Metals and Materials, University of Science and Technology Beijing, Beijing 100083, China
c Department of Chemistry and Biochemistry,The City college of New York, 160 Convent Ave., New York, NY 10031.
* Corresponding author. Tel. +86 22 60204909. Email:[email protected]
Abstract
  A facile one-step method for preparing nickel/cobalt layered double hydroxides (Ni/Co LDH) in a mixture of NH4Cl/NaOH solutions in the temperature range 45-85 oC is introduced. Ni/Co LDH consists of ultrathin nanosheets of various thicknesses assembled into porous nanostructures/microspheres. The mixed solution provides OH–, whose amount depends on the temperature, for reactions with Ni2+/Co2+ leading to the formation of hydroxides. The sample prepared at 70 °C shows the highest capacitance of 1142 F g-1 at a current density of 0.5 A g-1.That capacitance decreases about 15 % to 970 F g-1 at a high current density of 10 A g-1. The temperature effect on the arrangement of microsphere is apparent and Ni/Co LDH prepared at 70 oC has the most defectless texture (microspheres). The asymmetrical supercapacitor consisting of this sample as an anode and an activated carbon (AC) as a cathode works in a broad potential window from 0 to 1.6 V. The energy density of the Ni/Co of such a system reaches 58.13 Wh kg-1 at the power density of 160 W kg-1 owing to the intersectional nanosheets and unrestricted accessibility of electrolyte to the reaction centers. Even at a high discharge current density of 2 A g-1, the energy density still remains at 45.78 Wh kg-1 with a power density of 1600 W kg-1.
Keywords: mild temperature, layered double hydroxides, energy density, asymmetric supercapacitors.
1. Introduction
Nowadays, the development of efficient supercapacitors with high energy density is of paramount importance [1, 2]. Generally, supercapacitors can be classified into electrochemical double layer capacitors and pseudocapacitors [3]. In theory, pseudocapacitors exhibit a higher capacitance and energy density than the double layer capacitors. The latter stores electrical charge mainly at the electrode/electrolyte interface. However, the energy stored in supercapacitors is about 10 times smaller than that in batteries. This severely limits their applications where a high power density is required [4]. Therefore, there is a need to develop and to explore the advanced performance electrode materials for their applications in energy storage devices.
Get Help With Your Essay
If you need assistance with writing your essay, our professional essay writing service is here to help!
Layered double hydroxides (LDH), especially Ni/Co LDH compounds, are considered as materials of promising properties when applied in supercapacitors [5-7]. In their structure Co2+ can be oxidized to conductive CoOOH during redox reactions resulting in the improvement in conductivity of electrode materials [5]. For instance, Ni/Co LDHs hollow microspheres obtained in SiO2 templates exhibited the specific capacitance of 2171 F g-1 at the current density of 1 A g-1 [6]. Another examples are ultrathin NiCo-based LDH prepared at 120 °C, which displayed remarkable specific capacitance of 1537 F g-1 at 0.5 A g-1 [5]. It is important to mention that Ni/Co LDH referred as hydrotalcite-like compounds allow the intercalation of negatively charged anions within the interlayer space [7, 8]. This is expected to enhance the capacitive performance. Thus Lee et al. synthesized α-Ni(OH)2 through exchange of intercalated dodecyl sulfate anions by smaller anions (Cl–, NO3–, OAc– and SO42-). They found that larger anions hindered the mobility of OH– ions toward the surface of Ni(OH)2 sheets and Cl– intercalated Ni(OH)2 exhibited excellent capacitance. Especially, the Cl– ions located in the interlayer space were found as increasing its hydration level and thus enhanced the facile exchange and transport of OH– ions [9]. Hu et al. reported the effect of intercalated anions on the size of an interlayer spacing, morphologies, and capacitive performance of four different α-cobalt hydroxides. Their results showed the Cl– intercalated sample as the one of the marked capacitance. The good performance was linked to its high proton affinity [10].
In this work, we introduce a wet chemical approach to prepare Cl– intercalated Ni/Co LDH. The synthesis is done below 85°C, which is the lower temperature than those reported in other hydrothermal methods [11, 12]. In designing the method, it was expected that OH– ions from the solution of NH4Cl/NaOH will reacts with Ni2+/Co2+ to generate Ni/Co LDH. An important aspect is that the amount of the released OH– ions should be affected by the temperature. Thus the synthesis temperature is a factor, which we expect to govern the nanosheets assembling process and thus the final morphology of the materials. The effect of that morphology on capacitive behavior is investigated.
2. Experimental
2.1 Preparation of Ni/Co-LDH microspheres
All reagents were of analytical grade and were used without further purification. In a typical synthesis process, 3.75 mmol of Co(NO3)2·6H2O and 3.75 mol of Ni(NO3)2 6H2O were dissolved in 20 ml of water to form a pink solution (Solution A). 40 mmol of NH4Cl was added in 0.076 mol L-1 NaOH (180 mL) to form a transparent solution (Solution B); Then, the solution A was poured into 180 ml of the solution B with the final pH value of 9, in which the metal hydroxide mixtures were obtained. After stirring for 5 minutes, the solution was sealed in a glass bottle and transferred into an oven kept at a predetermined temperature (40, 55, 70 and 85 °C) for 15h. After cooled at room temperature, the obtained Ni/Co-LDH samples were washed with ethanol and water several times and dried in an oven at 60 °C for 12 h. The samples are referred to as Ni/Co LDH-x (x represents the reaction temperature of either 40, 55, 70 or 85 °C).
X-ray diffraction (XRD) patterns were measured on a Bruker D8 Advance diffractometer operated at 40 kV and 40 mA, Cu Kα radiation source (λ≈ 0.154 nm) was used. Fourier transform infrared spectroscopy (FT-IR) experiments were carried out on Bruker Vector 22 infrared spectroscopy in the range of 400 to 4000 cm-1with KBr tablets. Scanning electron microscopy studies (SEM) were performed on JSM-6490LV field emission scanning electron microscope. The sample was dispersed on the conductive tape fixed on the aluminum support. Transmission electron microscope (TEM) analysis was performed on a FEI Tecnai G20 microscope at 200 keV. The elemental analysis of as-prepared powder carried out using X-ray fluorescence spectrometer (XRF, CIT-3000SL).
2.3 Electrochemical tests
To evaluate the electrochemical performance of the synthesized materials, thee Ni/Co LDH samples were mixed with acetylene black and polytetrafluoroethylene (PTFE) in a mass ratio of 75:20:5. Homogeneous pastes obtained in ethanol were coated on a nickel foam substrate. The coated nickel foam was then pressed at 10 MPa and the obtained plate was used as a working electrode after dried at 60 °C for 12 h. The cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance (EIS) of the as-obtained samples were carried out on a IM6 & ZENNIUM electrochemical workstation in a three-electrode cell. The mass of working electrode was about 8 mg. The capacitance was measured in 6M KOH electrolyte using a platinum wire as a counter electrode and Ag/AgCl as a reference electrode. EIS tests were conducted in a frequency range from 100 kHz to 10 mHz with alternating current oscillation of 5 mV. The electrochemical performance of an asymmetric supercapacitor was investigated in a two-electrode cell of coin-type (CR2025), in which the prepared materials were used as a positive electrode and activated carbon- as a negative electrode. The experiments were carried out on the Land-CT2001A battery system in the potential range from 0 to 1.6 V.
3. Results and Discussion
3.1. Characterization of materials
The typical powder X-ray diffraction (XRD) patterns provide the information on the degree of crystallinity and the crystal structure of the Ni/Co LDH materials (Figure 1a) The distinct characteristic diffraction peaks on XRD patterns at 2θ 11.1, 22.2, 33.4 and 38.5 are indexed as (003), (006), (009) and (015) plane reflections of the hydrotalcite-like LDH phase, respectively [11, 13]. Increasing the reaction temperature leads to a slight increase in the intensity of the diffraction peaks. According to the Bragg equation, the calculated interlayer space of Ni/Co LDH is 0.79 nm. Ma et al. reported that the layered transitional metal hydroxides nanocones displayed a variety of interlayer space sizes depending on the kind of intercalated ions [12]. The values were 0.8 nm for NO3–, 0.92 nm for ClO4–, and 0.79 nm for Cl–. Our results suggest that Cl– ions are present in the interlayer space of the synthesized α-phase compounds.
FT-IR spectra of all samples are presented in Figure 1b. The broad band at about 3449 cm-1 is characteristic of the stretching vibration of hydroxyl groups hydrogen-bond to H2O [1]. The band at about 1630 cm-1 represents the bending mode of water molecules [10]. For all samples, three bands at 1476, 1349 and 854 cm-1 are linked to the typical features of carbonate ions [14]. Their presence is linked to the reactivity of hydroxides with atmospheric CO2. Bands below 800 cm-1represent stretching modes of metal-oxygen in the hydrotalcite-like lattice [1]. The band at about 649 cm-1 is assigned to Ni-OH bending. The absorption band at around 514 cm-1 represents the Co-O stretching vibrations [15]. The results indicate that the synthesized materials are nickel and cobalt layered double hydroxides.
Scanning electron microscopy (SEM) was used to analyze the morphologies of the obtained Ni/Co LDH materials prepared at different temperatures. Figure 2a shows a typical SEM image of Ni/Co LDH-40. There microspheres with a size around 3 μm and a small amount of aggregated nanosheets is visible (Fig. 2a inset). The micro-spherical particles consist of numerous interconnected nanosheets with thickness of about 30 nm. The morphology of Ni/Co LDH-55 presented in Figure 2b resembles that of Ni/Co LDH-40. The nanosheets with thickness around 20 nm are gathered in 3-D arrangements (Fig. 2b inset). When the reaction temperature increases to 70 °C, the microspheres of sample Ni/Co LDH-70 become well defined,defectless and uniform (Fig. 2c). Their size distribution is narrow with maximum at 3 μm. The detailed features of Ni/Co LDH-70 are shown in the Figure 2c (inset) where high-quality 3D flower-like microspheres are visible. The thickness of the nanosheets is about 15 nm. When the Ni/Co LDH material was prepared at 85 °C, the microspheres are not well-defined and some debris are visible (Fig. 2d). The spherical particles have a loose structure and some collapsed nanosheets overlay each other to construct larger sheets. Enlargement of the flower-like microsphere indicates that the nanosheets are interconnected and well-organized in three dimension (Fig. 2d inset). The thickness of nanosheets increases slightly when the temperature increases from 70 °C to 85 °C.
 In order to confirm the defectless microsphere/nanosheet morphology of Ni/Co LDH-70 sample, its internal structure has been further investigated by TEM. Figure 3a shows that the microsphere is assembled from nanosheets and the diameter of the microsphere is around 3 μm. The internal structure of microsphere is spacious and the nanosheets are the aggregates of even thinner layers. X-ray fluorescence (XRF) spectra were recorded for all samples. On the spectra, three marked peaks at 6.93, 7.48 and 8.26 KeV are identified as Co Kα, Ni Kα and Ni Kβ, respectively. This supports the co-existence of Ni and Co elements in all samples. Moreover, a peak at about 2.62 KeV is assigned as Cl Kα, which originates from the interlayer anion Cl–. It is interesting that the Ni/Co ratio of the as-obtained samples increased gradually with an increase in the temperature. This may be linked to the differences to the solubility constants of hydroxides (Ksp(Co(OH)2)>Ksp(Ni(OH)2)) and to the effect of temperature on that solubility [11].
 The results obtained suggest that the reaction temperature plays a crucial role in the formation of microspheres. The nucleation of Co(OH)2/Ni(OH)2 took places upon the addition of metal ions to the NH4Cl/NaOH solution. In such a system the involvement of OH– in the formation of metal hydroxides competes with the formation of ammonia (NH4Cl+ NaOH →NH4+ + OH– + NaCl →NH3 + H2O, Co2+ + OH– →Co(OH)2, Ni2++ OH– →Ni(OH)2). This affects the amount of OH reacting with metal ions. The size of nanosheets/hydroxide units increases with an increasing temperature owing to more energy provided to the system. This leads to a gradual increase in the sizes of microspheres built of these nanosheets. Even though the temperature range of the alkaline solution was not very broad and between 40 and 85 °C, 70 °C was found as the only temperature resulting in the uniform and defectless microspheres. Lower temperature of the system has an adverse effect on the formation of microspheres, likely due to the thicker nanosheets than those formed at 70 oC. On the other hand, higher temperature likely leads to collapsing of the formed microspheres [16].
3.2 Electrochemical tests
To evaluate the electrochemical performance of the synthesized materials, the cycling voltammetry (CV) curves were measured for Ni/Co LDH materials at a scan rate of 5 mv s-1 in 6M KOH aqueous solution (Fig. 4a). It is clearly seen that all curves exhibit a pair of redox peaks. An anodic peak is around 0.45 V and a cathodic peak- at about 0.2 V. They represent typical features of Faradaic pseudocapacitance representing the following reactions [6, 17].
Ni(OH)2 + OH– ↔ NiOOH +H2O +e-     (1)
Co(OH)2 + OH– ↔ CoOOH+H2O+e-      (2)
CoOOH+OH– ↔ CoO2+H2O+e-           (3)
The shapes of the CV curves indicate that Ni/Co LDH-70 has the highest specific capacitance [18].
To further investigate the capacitive behavior of our electroactive materials, the galvanostatic charge-discharge (GCD) measurements were performed in a three-electrode system and the specific capacitance (Cs) was calculated from the following equation (4):
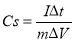 (4)
(4)
Where I is a constant discharge current (A), Δt is discharge time (s), ΔV is the potential difference, m is the mass of active material.
The GCD measurements in a potential window of 0-0.5 V are recorded at a current density of 1 A g-1 (Fig. 4b). The results show that the discharge time of Ni/Co LDH-70 is much longer than those of other electrodes. Based on the GCD curves, the specific capacitance was calculated. The values are 777, 608, 114,2 and 1017 F g-1 for Ni/Co LDH-40, Ni/Co LDH-55, Ni/Co LDH-70, and Ni/Co LDH-85, respectively. Wang et al. [19] prepared Ni/Co nanoplates using a surfactant assisted method. The rough nanoplates of an increased surface area displayed the specific capacitance of 311 F g-1, which was 2.3 times higher than that of smooth-surfaced Ni/Co nanoplates. It was concluded that creating more interspaces or a larger area can improve an electrochemical performance [19, 20]. Amongst our samples, Ni/Co LDH-70 with three dimensional defectless microspheres exhibits the highest capacitance. This texture promotes the electrolyte access to the more active centers than those available to electrolyte in more defected microspheres of the samples synthesized at other temperatures.
Find Out How UKEssays.com Can Help You!
Our academic experts are ready and waiting to assist with any writing project you may have. From simple essay plans, through to full dissertations, you can guarantee we have a service perfectly matched to your needs.
View our academic writing services
Since Ni/Co LDH-70 exhibits the best performance, its CV curves were measured at the scan rates 5, 10, 20, 50 and 100 mV s-1 (Figure 4c). The shape of the CV curves remains almost unchanged with an increase in the scan rate, suggesting a quick CV response with fast potential sweep and promising capability for energy storage [21]. In addition, all CV curves present a well-defined reduction and oxidation peaks with a slight shift in both cathodic and anodic peak potentials with an increasing scan rate. This suggests that an ion diffusion rate limits the redox reaction [22].
GCD curves of Ni/Co LDH-70 at different current densities (Fig.4d) show two visible voltage plateaus in the charge and discharge parts of the plots. More importantly, the GCD curves of Ni/Co LDH-70 are highly symmetrical at various current densities, indicating fast and good electrochemical reversibility of the Faradaic redox reactions. The capacitance of Ni/Co LDH-70 calculated from the GCD curves at the current densities of 0.5, 1, 2, 3, 5, 8 and 10 A g-1are 1142, 1130, 1098, 1081, 1053, 994, and 970 F g-1, respectively. The capacitance values gradually decrease with increasing current densities from 0.5 to 10 A g-1, owing to the fast electron transfer [23]. Ni/Co LDH-40, Ni/Co LDH-55 and Ni/Co LDH-85 exhibit the capacitance of 777, 608 and 1017 F g-1, respectively. It is important to mention that the specific capacitance of Ni/Co LDH-70 is 970 F g-1 at a high current density of 10 A g-1, and it consists of 84.9% of that at 0.5 A g-1. For Ni/Co LDH-40, Ni/Co LDH-55 and Ni/Co LDH-85, the corresponding values are at 610, 392 and 812 F g-1 representing the capacitance retentions of 78.5%, 64.5% and 79.8%, respectively. Such differences in the electrochemical performance can be explained by the morphology differences between the samples. As discussed above, the Ni/Co LDH-70 sample consists of uniform three dimensional microspheres built of nanosheets with the interspaces between them. These spaces increase the electrolyte access to the surface of the active materials. Thus the interconnected thin nonosheets in defectless microspheres provide more active sites for reactions with the electrons, which leads to the high capacitance [24, 25].
Electrochemical impedance spectroscopy (EIS) measurement is one of the fundamental methods to examine the electrochemical behavior of electrode materials. The Nyquist plots for the Ni/Co LDH materials in a frequency range of 100 kHz to10 mHz are displayed in Figure 5a. All the impedance spectra of the Ni-Co LDH materials are very similar to each other with a small semicircle at high-frequency and an inclined line at low-frequency [26]. The point intersecting with the real axis (Z’) at the high frequency represents the internal resistance, which includes the total resistances of intrinsic resistance of active materials, electrolyte resistance. and the contact resistance at electrolyte/electrode interface (Fig. 5a inset) [27]. This value is almost the same for all electrodes. The semicircle at the high frequency corresponds to the charge transfer resistance (Rct) [28]. At low frequencies, the liner part shows the Warburg impedance resulting from the ion diffusion resistance of the electrolyte in the active material [21]. Ni/Co LDH-70 exhibits the smallest semicircle diameter, which suggests the minimum charge transfer resistance and maximal electrochemical conductivity. The straight line at low frequencies range is close to Z” axis. This suggests that the capacitive performance is not only related to the diffusion process but double layer can also form [21, 29]. The effective ions diffusion and electron transfer of the Ni/Co LDH-70 electrode can be linked to the well-organized three dimensional and defectless micropheres [30].
For the evaluation of the feasibility of practical application of our materials, we constructed the asymmetric supercapacitors with Ni/Co LDH-70 as a positive electrode and activated carbon (AC) as a negative electrode in 6 M KOH solution. The charge-discharge measurements of the Ni/Co LDH-70 were carried out at the current density range from 0.2 to 2 A g-1 with an operating potential from 0 to 1.6 V (Fig. 5b). All curves show the symmetric-like shape, indicating the high electrochemical reversibility of the process. In addition, Ni/Co LDH-70 displays a small voltage drop at the beginning of the discharge process due to the internal resistance [31]. The values of specific capacitance of Ni/Co LDH-70 calculated from the galvanostatic charge -discharge curves are 163.5, 151, 137, 133 and 129 F g-1 at current densities of 0.2, 0.5, 1, 1.5 and 2 A g-1, respectively. And 78.7% of the capacitance is retained when the current density increases from 0.2 to 2 A g-1. For comparison, the specific capacitances of all supercapacitors tested are collected in Table 2. The supercapacitors of Ni/Co LDH-40, Ni/Co LDH-55 and Ni/Co LDH-85 show the specific capacitance of only 109, 86 and 150 F g-1 at 0.2 A g-1. They still keep 84, 62 and 117 F g-1 at 2 A g-1, respectively. The capacitances of Ni/Co LDH-40, Ni/Co LDH-55 and Ni/Co LDH-85 maintain at 77.2%, 72.3% and 78.2%, respectively, when the current density increases from 0.2 to 2 A g-1.
Based on those specific capacitance values, the energy and power densities of supercapacitors were further calculated (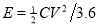 and
and 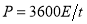 )[32]. Figure 5c shows that the energy densities decrease gradually with the increase in the power densities for all cells. At the same power density, the energy density of Ni/Co LDH-70 is much higher than those of other cells, which means that the Ni/Co LDH-70 cell has superior performance in terms of both energy and power density. The energy density of the Ni/Co LDH-70 is 58.13 Wh kg-1 at the power density of 160 W kg-1. Even at a high discharge current of 2 A g-1, the energy density still remains at 45.78 Wh kg-1 at a power density of 1600 W kg-1. Compared to other samples, the asymmetric supercapacitor of Ni/Co LDH-70 exhibits a significant improvement in both power density and energy density. This may be due to its broad potential window of 0-1.6 V and the high specific capacitance. The well-defined three dimensional microspheres of Ni/Co LDH-70 lead to the excellent energy-power combination. The results are comparable with those reported in the literature [5, 6]. For instance, Li et al. reported a new synthetic method for preparation of 16 nm-ultrathin NiCo-based layered double hydroxides (LDH) in an ethylene glycol solution. Their assembled asymmetric capacitor exhibited an energy density of 33.7 Wh kg-1 at power density of 551 W kg-1 with a 1.5 V operating voltage [5]. Tang et al. synthesized nickel cobalt double hydroxide nanoflowers with various Ni/Co ratios using a simple hydrothermal process at 120 °C. Their asymmetric supercapcitor exhibited 19.4 Wh kg-1 at 80.5 W kg-1, and even 20.6 Wh kg-1 at 3.93 kW kg-1 [6]. Cycling stability is a prime factor for evaluating the practical performance of asymmetric supercapacitors. Figure 5d collects the capacity for 2000 cycles of charge-discharge measurements between 0 and 1.6 V at a current density of 1 A g-1 in a two-electrode system. The capacitances of all Ni/Co LDH remains almost unchanged. With an increasing cycle number, the interfacial contact between the active material and a nickel foam may deteriorate, owing to the volume change of the active material during charge-discharge process. This might lead to a higher ohmic resistance of the asymmetric supercapacitors [33]. The calculated capacitances from GCD curve retain about 84.7%, 88.4%, 92.2% and 91.5% of their original capacitances after 2000 cycles for Ni/Co LDH-40, Ni/Co LDH-55, Ni/Co LDH-70 and Ni/Co LDH-85, respectively. The asymmetric supercapacitor of the Ni/Co LDH-70 shows a better cycling stability than those reported for other supercapacitors consisting of Ni/Co hydroxides [5, 6].
)[32]. Figure 5c shows that the energy densities decrease gradually with the increase in the power densities for all cells. At the same power density, the energy density of Ni/Co LDH-70 is much higher than those of other cells, which means that the Ni/Co LDH-70 cell has superior performance in terms of both energy and power density. The energy density of the Ni/Co LDH-70 is 58.13 Wh kg-1 at the power density of 160 W kg-1. Even at a high discharge current of 2 A g-1, the energy density still remains at 45.78 Wh kg-1 at a power density of 1600 W kg-1. Compared to other samples, the asymmetric supercapacitor of Ni/Co LDH-70 exhibits a significant improvement in both power density and energy density. This may be due to its broad potential window of 0-1.6 V and the high specific capacitance. The well-defined three dimensional microspheres of Ni/Co LDH-70 lead to the excellent energy-power combination. The results are comparable with those reported in the literature [5, 6]. For instance, Li et al. reported a new synthetic method for preparation of 16 nm-ultrathin NiCo-based layered double hydroxides (LDH) in an ethylene glycol solution. Their assembled asymmetric capacitor exhibited an energy density of 33.7 Wh kg-1 at power density of 551 W kg-1 with a 1.5 V operating voltage [5]. Tang et al. synthesized nickel cobalt double hydroxide nanoflowers with various Ni/Co ratios using a simple hydrothermal process at 120 °C. Their asymmetric supercapcitor exhibited 19.4 Wh kg-1 at 80.5 W kg-1, and even 20.6 Wh kg-1 at 3.93 kW kg-1 [6]. Cycling stability is a prime factor for evaluating the practical performance of asymmetric supercapacitors. Figure 5d collects the capacity for 2000 cycles of charge-discharge measurements between 0 and 1.6 V at a current density of 1 A g-1 in a two-electrode system. The capacitances of all Ni/Co LDH remains almost unchanged. With an increasing cycle number, the interfacial contact between the active material and a nickel foam may deteriorate, owing to the volume change of the active material during charge-discharge process. This might lead to a higher ohmic resistance of the asymmetric supercapacitors [33]. The calculated capacitances from GCD curve retain about 84.7%, 88.4%, 92.2% and 91.5% of their original capacitances after 2000 cycles for Ni/Co LDH-40, Ni/Co LDH-55, Ni/Co LDH-70 and Ni/Co LDH-85, respectively. The asymmetric supercapacitor of the Ni/Co LDH-70 shows a better cycling stability than those reported for other supercapacitors consisting of Ni/Co hydroxides [5, 6].
4. Conclusions
Ni/Co LDH of diverse morphologies determined by different particle sizes and thicknesses of nanosheets have been successfully prepared at various reaction temperatures in NH4Cl/NaOH mixture. Apparently the temperate affects the amount of OH– and the kinetic of its release. Both those factors govern the morphology of assemabled Ni/Co LDH. The synthesized materials showed differences in their electrochemical performance. The Ni/Co LDH-70 (prepared at 70°C) consists of uniform, defectless and well-defined microspheres with diameters around 3 μm. These 3-D microspheres are constructed from interconnected ultrathin nanosheets. Such an arrangement provides the space accessible to electrolyte and enables an efficient utilization of active centers. It also results in a high stability of the electrode upon cycling. Ni/Co LDH-70 shows the highest capacitance of 1142 F g-1 at a current density of 0.5 A g-1 and the value is still at 970 F g-1 at a high current density of 10A g-1, which is 84.9% of that at 0.5A g-1. The asymmetrical Ni/Co LDH-70 // activated carbon (AC) supercapacitor was tested in two-electrode system with a wide potential window of 0-1.6V. The results show that highest energy density of the Ni/Co LDH-70 reaching 58.13 Wh kg-1 at the power density of 160 W kg-1. Even at a high discharge current of 2 A g-1, the energy density still remains at 45.78 Wh kg-1 at a power density of 1600 W kg-1.
Acknowledgement
This work was supported by China Scholar Council, the Scientific Research Foundation for the returned overseas Chinese Scholars, State Education Ministry and Hebei Provincial Key Lab of Green Chemical Technology & High Efficient Energy Saving, School of Chemical Engineering Technology, Hebei University of Technology and Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Nankai University.
References
[1] T. Yan, Z.J. Li, R.Y. Li, Q. Ning, H. Kong, Y.L. Niu, J.K. Liu, Nickel-cobalt double hydroxides microspheres with hollow interior and hedgehog-like exterior structures for supercapacitors, Journal of Materials Chemistry, 22 (2012) 23587-23592.
[2] H.N. Ma, J. He, D.B. Xiong, J.S. Wu, Q.Q. Li, V. Dravid, Y.F. Zhao, Nickel Cobalt Hydroxide @Reduced Graphene Oxide Hybrid Nanolayers for High Performance Asymmetric Supercapacitors with Remarkable Cycling Stability, Acs Applied Materials & Interfaces, 8 (2016) 1992-2000.
[3] W.P. Sun, X.H. Rui, M. Ulaganathan, S. Madhavi, Q.Y. Yan, Few-layered Ni(OH)(2) nanosheets for high-performance supercapacitors, Journal of Power Sources, 295 (2015) 323-328.
[4] T. Yan, H.Y. Zhu, R.Y. Li, Z.J. Li, J.K. Liu, G.L. Wang, Z.Q. Gu, Microwave synthesis of nickel/cobalt double hydroxide ultrathin flowerclusters with three-dimensional structures for high-performance supercapacitors, Electrochimica Acta, 111 (2013) 71-79.
[5] R. Li, Z. Hu, X. Shao, P. Cheng, S. Li, W. Yu, W. Lin, D. Yuan, Large Scale Synthesis of NiCo Layered Double Hydroxides for Superior Asymmetric Electrochemical Capacitor, Scitific Report, 6 (2016) 18737.
[6] Y. Tang, Y. Liu, S. Yu, W. Guo, S. Mu, H. Wang, Y. Zhao, L. Hou, Y. Fan, F. Gao, Template-free hydrothermal synthesis of nickel cobalt hydroxide nanoflowers with high performance for asymmetric supercapacitor, Electrochimica Acta, 161 (2015) 279-289.
[7] B. Mavis, M. Akinc, Three-component layer double hydroxides by urea precipitation: structural stability and electrochemistry, Journal of Power Sources, 134 (2004) 308-317.
[8] T. Hibino, H. Ohya, Synthesis of crystalline layered double hydroxides: Precipitation by using urea hydrolysis and subsequent hydrothermal reactions in aqueous solutions, Applied Clay Science 45 (2009) 123-132.
[9] J.W. Lee, J.M. Ko, J.-D. Kim, Hierarchical Microspheres Based on alpha-Ni(OH)2 Nanosheets Intercalated with Different Anions: Synthesis, Anion Exchange, and Effect of Intercalated Anions on Electrochemical Capacitance, J Phys Chem C, 115 (2011) 19445-19454.
[10] Z.A. Hu, Y.L. Xie, Y.X. Wang, L.J. Xie, G.R. Fu, X.Q. Jin, Z.Y. Zhang, Y.Y. Yang, H.Y. Wu, Synthesis of alpha-Cobalt Hydroxides with Different Intercalated Anions and Effects of Intercalated Anions on Their Morphology, Basal Plane Spacing, and Capacitive Property, J Phys Chem C, 113 (2009) 12502-12508.
[11] H. Chen, L. Hu, M. Chen, Y. Yan, L. Wu, Nickel- Cobalt Layered Double Hydroxide Nanosheets for High- performance Supercapacitor Electrode Materials, Advanced Functional Materials, 24 (2014) 934-942.
[12] X. Liu, R. Ma, Y. Bando, T. Sasaki, A General Strategy to Layered Transition-Metal Hydroxide Nanocones: Tuning the Composition for High Electrochemical Performance, Advanced Materials, 24 (2012) 2148-2153.
[13] J. Huang, T. Lei, X. Wei, X. Liu, T. Liu, D. Cao, J. Yin, G. Wang, Effect of Al-doped beta-Ni(OH)(2) nanosheets on electrochemical behaviors for high performance supercapacitor application, Journal of Power Sources, 232 (2013) 370-375.
[14] M. Li, K.Y. Ma, J.P. Cheng, D.H. Lv, X.B. Zhang, Nickel-cobalt hydroxide nanoflakes conformal coating on carbon nanotubes as a supercapacitiv
Cite This Work
To export a reference to this article please select a referencing style below:
Give Yourself The Academic Edge Today
- On-time delivery or your money back
- A fully qualified writer in your subject
- In-depth proofreading by our Quality Control Team
- 100% confidentiality, the work is never re-sold or published
- Standard 7-day amendment period
- A paper written to the standard ordered
- A detailed plagiarism report
- A comprehensive quality report
Essay Writing Service
Essay Writing
Service
AED558.00
Approximate costs for Undergraduate 2:2
1000 words
7 day delivery
Order An Essay TodayDelivered on-time or your money back

1858 reviews

