ABSTRACT
In β-elimination reactions, the double bond is formed due to removal of β-hydrogen and the leaving group. The protonation of leaving group followed by ejection, formation of carbocation and abstraction of β-hydrogen successively to form alkene occur in elimination unimolecular (E1) reaction. In this experiment cycloalkene has been synthesized by heating respective cycloalkanol in water and catalytic amount of sulfuric acid. It was then distilled, dried with saturated sodium chloride and calcium chloride. Further distillation of dried organic layer gave product which was cyclopentene as analyzed with 13C NMR.
INTRODUCTION
The reactions in which β-hydrogen is eliminated making pi-bond followed by removal of leaving group are called β-elimination reactions. These reactions can be of E2, E1cb or E1 type. Elimination bimolecular (E2) reactions occur in antiperiplaner fashion in single step (concerted mechanism). These involve two reactants in rate determining step (RDS; single step) so they have second order kinetics. Strong bases for-example OH- and RO- favor E2 reaction. Elimination unimolecular conjugate base (E1cb) reactions take place in two steps. It takes place in basic condition when the hydrogen to be abstracted is relatively acidic (e.g. near carbonyl) and the anion so formed can be stabilized followed by the removal of poor leaving group. Elimination unimolecular (E1) reaction is stepwise reaction that involves carbocation formation. This is the slow rate determining step so exhibits first order kinetics. Tertiary carbocation formation and good leaving groups favor this reaction. It is regioselective and so more substituted alkene (Zaitsev’s rule) is predominantly formed.
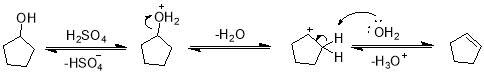
This experiment involves elimination unimolecular (E1) reaction since sulfuric acid first protonates the OH of alcohol forming it a good leaving group (as water) which then leaves forming carbocation. Water molecule then acts as base and abstracts β-hydrogen leading to the formation of alkene. Following are some applications of this reaction.
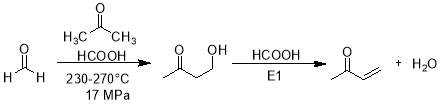
Catalyst free synthesis of 4-hydroxy-2-butanone under supercritical conditions at 230-270 ᵒC range with residence time 0.5-8 minute was conducted in tubular flow type reactor using formaldehyde (HCHO) and acetone (CH3)2O. A catalytic amount of formic acid (HCOOH) is produced through noncatalytic self-disproportionation of HCHO under these conditions which catalyze the synthesis and then dehydration of 4-hydroxy-2-butanone by E1 mechanism [1].
Some metal oxide nanoparticles have been prepared directly by the reaction of tert-amyl alcohol with metal oxides. These oxides or hydroxide include TiO2 nanoparticles and nanorods, SnO2 nanoparticles, Fe2O3 nanoparticles and FeOOH nanowires. Elimination unimolecular E1 has been considered to play main role due to stability of tertiary carbocation thus formed. Moreover, facile dissociation of C-O bond of tert-amyl alcohol is resulted due to strong interaction between hydroxide and metal chloride as shown below [2].

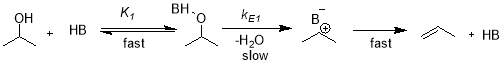
The dehydration of isopropanol at gas solid interface in continuous flow fixed-bed reactor at 0.94-5.52 kPa of isopropanol partial pressure and 55-135 ᵒC temperature range was carried out over various bulk Bronsted acid catalyst (HB). These catalysts were based on H3PW12O40 (HPW) Keggin-type heteropolyacid and Cs acid salt CsnH3-nPW12O40. The dehydration was believed to occur through E1 elimination pathway due to strong H+ sites on surface present on Keggian unit at peripheral oxygen atoms [3].
MATERIAL AND METHODS
|
EQUIPMENTS
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
REAGENTS
|
||
|
|
|
|
|
|
The equipment and reagents were provides by the instructors and purchased from commercial source by the chemistry department.
DISCUSSION
Elimination unimolecular (E1) reaction conditions have been employed in this experiment to obtain cycloalkene from corresponding cycloalkanol. Some drops of concentrated sulfuric acid were added to flask containing alcohol and water and heated for 30-40 minutes. Conc. Sulfuric acid first protonated the hydroxyl group making it good leaving group. Water as leaving group ejected forming an intermediate carbocation. The abstraction of β-hydrogen by water as base followed by the formation of double bond led to the formation of alkene.
It was then distilled till 0.5 mL sample was left in round bottom flask or 1.5 mL of the distillate was collected in small Erlenmeyer flask surrounded by ice. The distillation assembly was disassembled and cleaned for later use. The distillate (organic layer) was added to separatory funnel and washed and dried with equal amount of saturated aqueous sodium chloride solution. The organic layer was further dried with calcium chloride pellets that turned it transparent. Then it was distilled without using air condenser and collected in large preweighed vial placed in ice. The 13C-NMR of this product was obtained and analyzed.
Get Help With Your Essay
If you need assistance with writing your essay, our professional essay writing service is here to help!
Three peaks in 13C-NMR spectra suggested the presence of three kinds of carbons in the product. Among those two peaks at the positions 19.6 and 31.6 ppm showed the presence of CH2 groups where peak at 31.6 ppm belonged to allylic CH2. Peak at 130.2 ppm suggested the occurrence of CH (alkene). Furthermore the molecular weight 68.1 g/mol indicated that this spectra belonged to cyclopentene. All these observations confirmed that the product was cyclopentene and the starting alcohol was cyclopentanol.

THEORETICAL YIELD
86.13 g cyclopentanol yields cyclopentene = 68.11g
1 g cyclopentanol yields cyclopentene = 68.11 / 86.13
2 g cyclopentanol yields cyclopentene = (68.11 / 86.13) x 2
= 1.58 g
REAGENT TABLE:
|
Compound |
Molecular Weight (g/mol) |
Structure |
Boiling point (ᵒC) |
Melting point (ᵒC) |
Density (g/cm3) |
Number of moles (mol) |
Weight (in grams) |
|
Cyclopentanol |
86.13 |
|
140.4 |
-19 |
0.949 |
2/86.13 = 0.0232 |
2g |
|
Cyclopentene |
68.11 |
|
44 |
-135 |
0.744 |
N/A |
N/A |
|
Conc. Sulfuric acid |
98.079 |
|
337 |
10 |
1.84 |
- |
- |
|
Water |
18 |
|
100 |
0 |
0.997 |
0.01661 |
0.2991 |
|
Sodium chloride |
58.44 |
|
1465 |
801 |
1.202 (saturated solution) |
- |
- |
|
Acetone |
58.08 |
|
56 |
-95 |
0.784 |
- |
- |
|
Calcium chloride |
110.98 |
|
1935 |
772 |
2.15 |
- |
- |
EXPERIMENTAL
To round bottom flask (10 mL) a boiling chip, water (0.3 mL), concentrated sulfuric acid (5 drops) and unknown alcohol (2.0 g) were added and swirled. Air condenser was fitted and mixture was gently heated on heating mantle for 30-40 minutes. Distillation apparatus was assembled with it and distillation was performed till 0.5 mL of sample was left in flask. The distillate was collected in small Erlenmeyer flask placed in ice surrounding it. The temperature range of sample collection was recorded. The apparatus was removed from heat, allowed to cool and disassembled. It was washed, rinsed with water and then acetone and dried. The contents of small Erlenmeyer flask were poured into separatory funnel. Equal amount of saturated aqueous sodium chloride soln. was introduced to it, shaked, vent and drained the lower (aqueous) layer. The organic layer was drained to Erlenmeyer flask kept on ice, covered and dried for two minutes with calcium chloride pellets (cloudy solution got cleared due to water removal). The dried organic layer was then introduced to round bottom flask, reassembled the distillation apparatus without condenser and second distillation was performed very carefully (to avoid missing boiling point) because it occurred quickly. The distillate was collected in a pre-weighed (capped) large vial kept in ice. After distillation the vial was capped, dried from outside and weighed again. This was the weight of alkene. The 13C NMR sample was prepared and spectra were recorded.
13CNMR spectrum (CDCl3), δ, ppm: 19.6 (CH2); 31.6 (CH2 allylic); 130.2 (CH alkene)
CONCLUSION
In this experiment Elimination unimolecular (E1) reaction has been successfully applied to synthesize cycloalkene by the dehydration of corresponding cycloalkanol. The 13C NMR spectra and molecular weight suggest the product to be cyclopentene formed by successful dehydration of cyclopentanol.
REFERENCES
- Mei, J., Mao, J., Chen, Z., Yuan, S., Li, H., & Yin, H. (2015). Mechanism and kinetics of 4-hydroxy-2-butanone formation from formaldehyde and acetone under supercritical conditions and in high-temperature liquid-phase. Chemical Engineering Science, 131, 213-218.
- Hu, M. J., Gao, J., Yang, S., Dong, Y., Wong, J. S. P., Xu, J., ... & Li, R. K. (2012). E1 reaction-induced synthesis of hydrophilic oxide nanoparticles in a non-hydrophilic solvent. Nanoscale, 4(20), 6284-6288.
- Bond, G. C., Frodsham, S. J., Jubb, P., Kozhevnikova, E. F., & Kozhevnikov, I. V. (2012). Compensation effect in isopropanol dehydration over heteropoly acid catalysts at a gas–solid interface. Journal of catalysis, 293, 158-164.
Cite This Work
To export a reference to this article please select a referencing style below:









