Brendaliz Bonilla
Chemistry Department
Susquehanna University
Abstract
Esters are prepared in many ways and one of which is through Fischer Esterification. Using this method, esters are produced by refluxing a carboxylic acid and an alcohol in the presence of a concentrated acid catalyst.1 The purpose of reflux is to heat a reaction mixture at its boiling temperature to form products, without losing any of the compounds in the reaction flask. To exploit Le Chatelier’s principle and shift the position of the equilibrium to the right, an excess of one of the reactants were added to the reaction mixture.1 The reaction mechanism involves initial protonation of the carboxyl group, nucleophilic attack by the hydroxyl, proton transfer, and loss of water followed by loss of the catalyzing acid to produce the ester.2 The process is thermodynamically controlled yielding the most stable ester product. Typically, only primary and secondary alcohols are used in the Fisher method since tertiary alcohols are prone to elimination.3 In this lab, a Fisher Esterification was performed to synthesize isopentyl acetate from isopentyl alcohol and acetic acid as seen in figure 1.
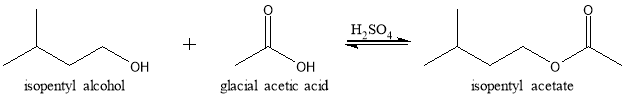
Figure 1: Reaction scheme of the preparation of isopentyl acetate by Fischer Esterification.
Experimental
Instruments Used: A Nicolet IR 100 FT-IR was used in this experiment.
Procedure and Observations: A mixture of 5.0mL (4.111g) of isopentyl alcohol, 7.0mL of glacial acetic acid, and 1mL of concentrated sulfuric acid was added to a 25mL round-bottomed flask. The round-bottomed flask was hooked to the reflux apparatus and the mixture was brought to a boil for an hour. The mixture was cooled to room temperature, placed into an ice bath, and put in a separatory funnel with 10mL of water. The funnel was shaken vigorously and vented several times. The bottom layer was drained from the separatory funnel into a beaker. 5mL of 5% sodium bicarbonate was then put into the separatory funnel. The separatory funnel was shaken and vented several times. The bottom layer was drained into the same beaker. 5mL of saturated sodium chloride was added to the contents of the separatory funnel. The separatory funnel was shaken and vented several times. The bottom layer was drained into a different beaker. The mixture that was left in the separatory funnel was transferred to an Erlenmeyer flask with 1g of anhydrous sodium sulfate. The flask was corked and was left to sit for 10 to 15 minutes. The mixture was transferred to another Erlenmeyer flask and .503g of anhydrous sodium sulfate was added. A distillation apparatus was assembled with the receiving flask immersed in an ice bath. The mixture was transferred into a round-bottomed flask and attached to the distillation apparatus. The product that was now in the receiving flask was then weighed. The percent yield was determined and an IR was done on the product.
Results and Discussion
At the end of the experiment, a successful esterification was performed from the starting acetic acid, using isopentenyl alcohol to make the product of Isopentyl acetate. The reactants were heated using a reflux apparatus so that the product would not be lost, helping serve as a catalyst in the reaction.1 Any remaining water left over from the esterification process was dried using anhydrous sodium sulfate. The ester, isopentyl acetate was synthesized, which had the smell of bananas. In this experiment, 3.99 g of isopentyl acetate was formed by the direct esterification of acetic acid with isopentyl alcohol, as seen in table 1. The sulfuric acid was used as a catalyst in the reaction.
Table 1: The weight of the final product collect, percent yield, and result of the IR spectrum.
|
Weight (grams) |
3.99 g |
|
Percent yield |
61.8% |
|
IR Peaks (cm-1) |
2954, 1747, 1231, and 1056 cm-1 |
An excess of isopentyl acetate was used to shift the reaction to the right so that esterification could occur. During isolation, the excess acetic acid and isopentyl alcohol was removed with sodium bicarbonate, and the isopentyl acetate was further purified after through drying with anhydrous sulfate and through distillation. The excess acetic acid was used in order for the reaction to favor esterification. An excess of isopentyl alcohol could have been used instead to form isopentyl acetate; however excess acetic acid is easier to remove from the products than isopentyl alcohol because isopentyl acetate and isopentyl alcohol are similar in structure and therefore, prefer to be in the same layer of the solution. Since sodium carbonate is a base, it is used in the extraction of acetic acid because it turns acetic acid into a conjugate base or sodium acetate which is more soluble in water. The equation for this acid-base extraction is: CH3COOH+NaCHO3→CH3COO-Na + H2CO3.
Find Out How UKEssays.com Can Help You!
Our academic experts are ready and waiting to assist with any writing project you may have. From simple essay plans, through to full dissertations, you can guarantee we have a service perfectly matched to your needs.
View our academic writing services
The percent yield of the isopentyl acetate was 61.9 % (as seen in table 1) with a theoretical yield of 6.44g. In the experiment, the acetic acid was in excess and the isopentyl alcohol was the limiting reagent, therefore, the reaction depended on the amount of isopentyl alcohol available. This experiment was successful because the smell of bananas was achieved along with the percent yield attained of 61.9%. Some of the errors that might have occurred included not properly/fully draining the aqueous layers after the reflux, and that the solution may have not completely dried with anhydrous sodium sulfate. For the IR spectrum data, the -C-CO 2R stretch characteristic of an ester is visible in the pure isopentyl acetate IR spectrum in the 1735-1745 cm -1 range. The -C-H stretches are visible just below 3000 cm -1, and the -C-O and -CO 2 stretches appear as several peaks in the 1050-1300 cm -1 range, which can be seen in Appendix E. The product resulted in major IR peaks at 2954, 1747, 1231, and 1056 cm-1. These results indicate that our isopentyl acetate product is very pure, as the peaks are nearly identical to the expected peaks. The peak at 2954 indicates the C-H bond. The peak at 1747 indicates the aldehyde (C=O). The peak at 1231 indicates methyl group. The peak at 1056 indicates residual acetic acid (R-Cl), which can be seen on Appendix D-G.
Conclusion
The major product that was formed from the Fischer Esterification of isopentyl alcohol and acetic acid was isopentyl acetate. This is because the ester formed is the equatorial position, which makes the compound more stable than cis-4-tert-butylcyclohexanol. Based on the experiment that was conducted the synthesis of isopentyl acetate from a carboxylic acid and an alcohol could be done by a Fisher Esterification reaction, and the percent yield of the product is about 61.9%.
References
- “Experiment 4 Background.” Experiment 4 Background. Web. Accessed: 15 Feb. 2017.
http://www.reed.edu/chemistry/alan/201_202/lab_manual/Expt_banana_oil/background.html.
- Mutual Solubility of Water and Aliphatic Alcohols.” Mutual Solubility of Water and Aliphatic Alcohols – Journal of Chemical & Engineering Data (ACS Publications). Web. Accessed: 15 Feb. 2017. http://pubs.acs.org/doi/abs/10.1021/je00037a019.
- “Alcohol Reactivity.” Alcohol Reactivity. Web. Accessed:17 Feb. 2017. https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/alcohol1.htm.
Appendix A: Finding the Limiting Reagent
Grams X 1 mol / molecular weight = moles of reactant
Glacial Acetic Acid:
8.5 mL X ((1 g/1 mL) X 1 mol) / 60.05 g/mol = 0.142 mol
Isopentyl Alcohol:
(4.37 g X 1 mol) / 88.15 g/mol = 0.0459 mol
Appendix B: Calculating Theoretical Yield of Isopentyl Acetate
(Moles of limiting reagent X molar ratio X molecular weight of product) / 1 mol = theoretical yield
(0.0459 X 130.19) / 1 mol = 6.44 g
Appendix C: Calculating Percent Yield
(Actual / theoretical) X 100% = percent yield
(3.99 g/ 6.44 g) X 100% = 61.9%
Cite This Work
To export a reference to this article please select a referencing style below:


