Introduction
Organic chemistry is the study of the structure, properties, composition, reactions, and preparation of carbon-containing compounds, that include compounds with any number of elements other than hydrocarbons. This branch of chemistry was originally limited to compounds produced by living organisms but has been broadened to include human-made substances such as alcohol. Alcohol is an organic compound in which the functional group hydroxyl (-OH) is bound to saturated carbon atom. In general usage, the most commonly used alcohol is ethanol (ethyl alcohol). This report will be about the use, disadvantages and the effect of ethanol on the environment and society. Ethanol has many different uses in people’s everyday life. Using it as an automotive fuel is the most common one therefore, it is the most widely used alcohol in society.
Chemical Structure
The formula structure of ethanol is (C2H5OH), as it is shown in (Figure1). It evident the hydrogen polar bond between the molecules.
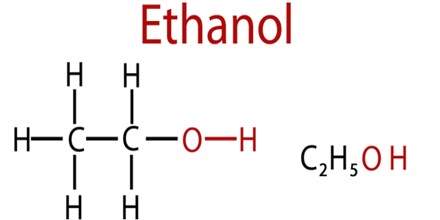
Figure1: the formula structure of ethanol.
Alcohol is classified according to the number of alkyl groups attached to the carbon bonded to the hydroxyl group. There are three types of alcohol which are primary, secondary and tertiary as it highlighted in (Figure2).

Figure2: classification of alcohols.
The properties of alcohols are determined by the presence of hydrogen bonds. All alcohols in general have high boiling points that increase as the size of the molecules increase. Also, because the dispersion forces between these gradually larger molecules also increase. The 3D structure of molecules can also affect the boiling point of a compound. Primary alcohol needs more energy to break it down than secondary and tertiary alcohol. This is because primary alcohol is more exposed that has strong intermolecular forces, which is more likely to form hydrogen bonds with other alcohols. Melting point also, follow the same concept as boiling point. The solubility of an alcohol depends on the strength of intermolecular interactions between the solute and the solvent. Alcohol becomes less soluble as the chain length increases which is due to the increase in the non-polar molecules.
Chemical Reactions Involved in Ethanol’s Production
Ethanol is a chemical that is volatile, colourless, flammable. Synthetic ethanol can also be produced from non-renewable sources like coal and gas. It can be produced from petroleum via chemical transformation of ethylene, but it can also be produced by fermentation of glucose, using yeast as it shown in (Figure3). Fermentation is the common way that use to form ethanol. Also, fermentation is the process in which yeast breaks down sugar into alcohol and carbon dioxide which is evidence in (Figure3). Yeast are tiny single-celled fungi that contain special enzymes responsible for this reaction. The fermentation reaction requires conditions such as temperature, substrate the glucose solution, absence of oxygen and yeast. The temperature must be between the range of 25°C and 50°C. Enzymes are affected a great deal by temperature. If the temperature is too cold the enzymes move around too slowly to meet the substrate and for a reaction to occur. As the temperature increases though, so does the rate of reaction. Enzymes work best when there is a high enough substrate concentration for the reaction they catalyse. If too little substrate is available, the rate of the reaction is slowed and cannot increase any further. In addition, air must be excluded from the vessel in which fermentation is being carried out. Air contains a large proportion of bacteria called Acetoacetic. Acetoacetic bacteria use atmospheric oxygen from air to oxidise ethanol in the wine, producing a weak solution of ethanoic acid (vinegar). With temperature held between 15-35°C, however the fermentation process stops when ethanol concentration is above 15% as bacteria and yeast cannot survive. To increase ethanol’s concentration, it needs to be separate by distillation.
 Figure3: production of ethanol.
Figure3: production of ethanol.
Safety Disposed of Ethanol
Because ethanol is highly flammable and combustible, there are a couple of safety measurements to take in order to safely protect the environment from the dangerous waste. Ethanol should be deposited as hazardous waste in the appropriate waste container. Solid waste having ethanol is to be disposed of into waste bins but the container containing ethanol should be labelled and stored separately to other acids or chemicals with no longer than 90 days and to rinse off the empty containers with water; dry and dispose capped in the glassware container or trash bin. However, when ethanol is disposed of incorrectly, that may cause an explosion that will has negative effect on the environment.
Find Out How UKEssays.com Can Help You!
Our academic experts are ready and waiting to assist with any writing project you may have. From simple essay plans, through to full dissertations, you can guarantee we have a service perfectly matched to your needs.
View our academic writing services
The Advantage and Disadvantage of Ethanol
Ethanol is a water-soluble liquid at room temperature, making it useful as solvents in cosmetics and pharmaceuticals, as well as being able to kill the microorganisms hence its used in hand sanitisers. Also, it is active ingredient in alcoholic beverages. The domestically produced transportation fuels for example E10 that has low level blends (10% ethanol, 90% gasoline), or in E85 (flex fuel) a gasoline-ethanol blend containing 51% to 83% ethanol. It is a clear, colourless liquid and the principle ingredient in alcoholic beverages like beer, wine or brandy, which made it the only alcohol that is drinkable. This is because it can readily dissolve in water and other organic compounds. Also, it enters the production of some products, from personal care and beauty products to paints and varnishes to fuel as mentioned above. Moreover, it can use as enhance the flavour of food extracts such as vanilla extract. Ethanol uses as a food additive, thus, evenly distribute food colouring, as well as enhance the flavour of food extracts. Ethanol is classified as a renewable resource because it’s mainly as a consequence of conversion of energy from the sun into useful energy. The production of ethanol begins with the photosynthesis process, which enables sugarcane to thrive and later be processed into ethanol fuel. Ethanol also, used as a sterile and effective antiseptic through the addition of some compounds that help to change the poison properties with ethanol. Lastly, ethanol helps in the production of vinegar and yeast.
Although, ethanol is used widely in different branches of industry and in everyday life. However, drinking large amount can have a bad effect on the person’s body such as vision disturbances and weakness of memory. It decreases the levels of sugar in the body with having a major impact on the other body systems including liver (where it is metabolised) leading to fatty liver disease besides high blood pressure. Moreover, it can damage the nerve and causes degeneration of the nervous system considering the alcohol is a solvent, it can penetrate well into fat tissues like the brain hence the damaging effects. Also, major environmental problems would arise out of the disposal of waste fermentation liquors. The primary disadvantage of ethanol is that it requires cropland space in which to grow. Ethanol is a less effective fuel than fossil fuels. Although pipelines could be used to carry ethanol throughout the country, most of them would need to be retrofitted. Ethanol is highly corrosive because it has an ability to absorb water. That makes it difficult to ship the fuel over long distances unless there are protective technologies incorporated into the distribution networks.
The Impact of Ethanol on the Environment
Ethanol production only creates few greenhouse emissions (30%) when compared to other fuels due to the improvement technology leading to a balance in positive energy. Burning ethanol causes less pollution to the environment and it reduces the dependency of oil. However, it has major disadvantages such as fertiliser, salinity, deforestation and soil erosion besides its waste that comes from fermentation liquors impacting the environment. Moreover, highly-concentrated ethanol leads to advanced modifications of various engines in order to operate properly. Finally, ethanol can reduce pollution. Ethanol has higher evaporative emissions from fuel tanks and dispensing equipment. These evaporative emissions contribute to the formation of harmful, ground-level ozone and smog.
The Economic and Social Cost
Ethanol is now the most widely used alternative fuel in the world however, the Australian ethanol industry is relatively small by world standards. The ethanol industry in Australia is quite small, in the order of 135 million litres. The use of fuel ethanol attracted considerable negative press and public comment in the later part of 2002. This is because people think that using more than 10% ethanol in fuel cause problems in cars. However, there is no serious proof from the government and scientists. Moreover, a major disadvantage of fuel ethanol is its production cost, which is around 70 cents a litre compared with around 35 cents per litre current world crude oil prices for unleaded petrol.
Conclusion/Recommendations
In conclusion, ethanol which is a type of alcohol is significant for individuals and the environment. It helps to produce wine, food additive, personal care and beauty products to paints and varnishes to fuel as mentioned above. However, it can damage the nerve and causes degeneration of the nervous system considering, and major environmental problems would arise out of the disposal of waste fermentation liquors. Moreover, ethanol has social and economic cost as the redirection of first express juices the primary sugar cane product to ethanol production would greatly increase the price of ethanol feedstock and hence the cost of producing ethanol; much make people unused to it. Therefore, I recommend the government take more using of ethanol as a fuel in serious way, which will assist to implementing the use of ethanol instead of fossil fuels that takes millions and billions of years to occur while ethanol is renewable. Also, ethanol is cheaper than fossil fuels as well as it is environmentally friendly not like fossil fuels that produce high amount of CO2 and cause increasing in greenhouse. Increasing the production of ethanol will creates jobs in rural and regional areas therefore, increased economic activity in regional areas.
Work Cited-Bibliography
- Fuel Ethanol-Background and Policy Issues – Parliament of Australia. (2019). Retrieved from https://www.aph.gov.au/About_Parliament/Parliamentary_Departments/Parliamentary_Library/Publications_Archive/CIB/cib0203/03cib12.
- The Process of Industrial Alcohol Production. (2019). Retrieved from http://www.articlesfactory.com/articles/business/the-process-of-industrial-alcohol-production.html.
- Ethanol Uses, Benefits, and Chemical Safety Facts. (2019). Retrieved from https://www.chemicalsafetyfacts.org/ethanol/.
- (2019). Retrieved from https://sites.chemengr.ucsb.edu/~ceweb/faculty/scott/Chemical%20SOPs/Ethanol.pdf.
- (2019). Retrieved from https://www.chemistryviews.org/details/ezine/10517511/The_Oxidation_of_Alcohols.html.
- HSC Chemistry – Production of Materials – dot point notes – Dux College. (2019). Retrieved from https://dc.edu.au/dot-point-summary-production-of-materials/.
- Advantages and Disadvantages of Ethanol. (2019). Retrieved from https://occupytheory.org/advantages-and-disadvantages-of-ethanol/ .
- 7.1 Ethanol Production – General Information | EGEE 439: Alternative Fuels from Biomass Sources. (2019). Retrieved from https://www.e-education.psu.edu/egee439/node/646.
- 11.2 Ethanol Production and Economics | EGEE 439: Alternative Fuels from Biomass Sources. (2019). Retrieved from https://www.e-education.psu.edu/egee439/node/720.
- (2019). Retrieved from https://www.cabdirect.org/cabdirect/abstract/20093342133.
- Sarathy, S., Oßwald, P., Hansen, N., & Kohse-Höinghaus, K. (2019). Alcohol combustion
chemistry.
- (2019). Retrieved from https://study.com/academy/lesson/alcohols-alkanols-classification-functional-ghttps://study.com/academy/lesson/alcohols-alkanols-classification-functional-group.htmlroup.html
- Ethanol and the Environment – Energy Explained, Your Guide To Understanding Energy – Energy Information Administration. (2019). Retrieved from https://www.eia.gov/energyexplained/index.php?page=biofuel_ethanol_environment
- Ayres, C. (2019). 11 Advantages and Disadvantages of Ethanol. Retrieved from https://vittana.org/11-advantages-and-disadvantages-of-ethanol
- Alcoholic fermentation, of sugar into CO2 and alcohol. (2019). Retrieved from https://www.yobrew.co.uk/fermentation.php
- Alternative Fuels Data Center: Ethanol Benefits and Considerations. (2019). Retrieved from https://afdc.energy.gov/fuels/ethanol_benefits.html
- Figure1: Ethanol Formula, Boiling, Melting Point, pH, Density, Solubility. (2019). Retrieved from http://www.nutrientsreview.com/alcohol/definition-physical-chemical-properties.html
- Figure2: Alcohols & Alkanols: Classification & Functional Group – Video & Lesson Transcript | Study.com. (2019). Retrieved from https://study.com/academy/lesson/alcohols-alkanols-classification-functional-group.html
- Figure3: How is ethanol made? – Biofuels Association of Australia. (2019). Retrieved from http://biofuelsassociation.com.au/biofuels/ethanol/how-is-ethanol-made/
Cite This Work
To export a reference to this article please select a referencing style below:


