The Importance of Chlorine
Exploring The Use Of Chlorine In Ecology, Chemistry, Astronomy, And Electricity

[This image displays a gas mask. This is because chlorine is a poisonous gas,and has been used as a chemical weapon.][15]
CHEMISTRY
Chlorine is one of the most sufficient, and frequently occurring elements. It has a critical role in the manufacturing of millions of products we use in an everyday basis.[1] Chlorine derivatives and chlorinated chemicals contribute to and are used in health care, transportation, building and construction, clothes, defense and law enforcement, and the food and water sectors. In fact, a recent economic analysis found in varied operations, U.S. and Canadian customer conserve over $421.5 billion per year by using chlor-alkali chemistry. In addition to that, statistically, at some period in their manufacturing, 88% of current pharmaceuticals adopted chlorine primary breakpoint chlorination.[7]
Chlorine gas has a toxic odour, and inhaling it causes choking, chest pain, tight throat, and has a long term effect of edema. Inhaling one part per million can cause detrimental effects; however, inhaling one part per thousand within minutes causes immediate death. Because of its severe reaction, chlorine was served in World War I in chemical warfare. [3]
Chlorine has one of the highest electronegativity, being slightly lower than fluorine; however, it has the highest electron affinity. More so that the affinity of chlorine for hydrogen is so extreme that it reacts with a violent explosion in light.[4] This is extremely good as hydrogen chloride is applied in the production of many industrial chemicals, such as fertilizers and dyes.
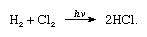
[ In the following equation where hν is light, it portrays chlorine high electron affinity with hydrogen][4]
In consideration of its structure, chlorine slightly dissolves in water. Its solubility is 0.7 with g/100 ml at 20°C. At -35°C, it liquefies and transforms from gas to liquid and it can also liquefy at room temperature if intense pressure is applied. At 0°C, the density of chlorine in liquid state is 1.47 g/cm3. It’s not flammable; however, like oxygen, can combust with the chemical combination of other substances.[4]
Get Help With Your Essay
If you need assistance with writing your essay, our professional essay writing service is here to help!
ASTRONOMY
Chlorine is a highly reactive gas. Such being the case, if chlorofluorocarbons are released in the air, the ozone layer in the earth’s atmosphere will be harmed, upon exposure to ultraviolet radiation. Fortunately, it has been proposed that the chlorine atom could be a significant reactive in the troposphere, as it is in the stratosphere, where it is responsible for ozone depletion. However, there are many adjustments that must be done in order to fulfill that and so it’s unlikely to occur.[5]
Recently, a company of Livingston & Wallace used the Phoenix infrared spectrometer to report chlorine abundances in cool stars; this includes 15 evolved giants and 1 M dwarf.[7]
At Barrow, Alaska, there is a relative importance of chlorine and bromine radicals in the oxidation of atmospheric mercury. It is investigated that the concentration of Br and Cl atom has a contribution to the observed decay of Gaseous Elemental Mercury (GEM) for five cases of apparent first‐order decomposition.[2]
Furthermore, in the surface of Mercury, chlorine has a role in the production, ascent, and eruption of flood volcanic material in this region. This is important because most of the particles spewed from volcanoes cool the planet by shading incoming solar radiation.[8]
Hydrazine-chlorine pentafluoride is a reaction that occurs in a laboratory rocket combustor and causes an attractive, high-energy, storable propellant. It is among the highest performance storable propellant because of a theoretical specific amount of impulse of 312sec.[9]
Since 1970, the Homestake Chlorine Detector, built by the inverse beta-decay reaction, has been calculating the flux of solar neutrinos. After linking the result of 108 extractions, they finally detected solar neutrino-induced 37Ar with a production rate of 2.56 ± 0.l6 (statistical) ± 0.16 (systematic) SNU.[18]
ELECTRICITY
Fiber optic technology is transforming the fast-changing world of communications by transferring digital information through hair-thin strands of ultra-pure glass. To manufacture high-purity fiber optic glass, chlorine chemistry is crucial. There are a lot of benefits optic fibers provides over traditional metal wires; greater energy efficiency, lower cost, a clearer signal and lower fire hazard.Furthermore, optical fibers transmit data faster, 10 gigabytes per second, than metals wire, 1.5 gigabytes per second.
Titanium dioxide is a semiconductor which is used to catalyse the photodecomposition of water into hydrogen and oxygen. The manufacture of titanium dioxide include the two main processes, the sulfate process and the chloride process, which include the conversion of rutile to titanium (IV) chloride, and the oxidation of titanium (IV) chloride.[10]
Using chlorine chemistry, polyurethane foam is produced to decrease energy bills and conserve natural resources, by the growth of energy efficiency of home heating and air-conditioning systems. Home heating, cooling costs, and fossil fuel energy-associated greenhouse gas emissions are also lessened because of energy-efficient polyvinyl chloride (PVC) windows. [11]
Chlorine chemistry has a significant impact in exploiting solar energy; to help transform silicon that was purified from the grains of sand into solar panel chips. For a renewable, sustainable, co-environmental greenhouse energy source, the rotary mechanical device, turbine, has blades of chlorine-based epoxy resins that helps to transform wind power into electricity.[12]
Polyvinyl Chloride is used for wire insulation, cable jacketing, UV resistant; because it exhibits good resistance to chemicals and water, and it’s durable. It is generally applied in OEM Markets, Appliance Wiring Industry, Medical Industry, Communications Industry, and Oil and Gas Industry as an insulation material.[14]
ECOLOGY
When chlorine, in gas or liquid state, escapes from industrial settings where it is used in large quantity, it has bad impacts on the environments. Through combination of chlorine with other species carried in ships’ ballast water, it causes disastrous effects on the marine ecosystem. However, a large portion of the presently authorized ballast water treatment systems that includes chlorine/hypochlorite as the disinfection agent is assigned for it and is slightly improving. [16]
The chloride ion is essential to life for all biological beings. It is mostly present in cell fluid as negative ions to balance the positive ions; such as potassium, and extracellular fluids.
Polyvinyl chloride (PVC) is comprised of 57 percent chlorine which is not similar to other plastics that are produced from fossil fuels. Because of chlorine, PVC can recycle aluminum beverage cans, and extract impurities in molten aluminum during the recycling process.[7]
During the process of chlorination where chlorine cleans the water and kills the germs. Living beings have drinkable water that does not permit health problems and provides protection against waterborne disease outbreaks. [17]
Chlorine chemistry is used to produce crop protection products; this includes compounds that are used as herbicides, insecticides, fungicides, nematicides, and growth regulators. Products with natural and synthetic fertilizers improve yields, reduce crop losses and soil erosion. It also produces high-quality products at the lowest possible cost to consumers. [13]
Oddly enough from what was said before, DDT, an insecticide in which has chlorine as an ingredient, was highlighted in Rachel Carson’s seminal work “Silent Spring,” as the cause for the decrease in bird population, and was banned all around the world.[7]
Additionally, overuse of chlorine is polluting alkalies and chemical plants at Ranoli, near Baroda, specifically on three tropical fruit trees; mango, rayan, and jamun. These trees have a higher accumulation of chloride in their foliar tissues, and they, growing close to the other plants, showed a high percentage of damaged leaf area, and an overall reduction in fruit yield. [13]
REFERENCES
- https://www.pnas.org/content/106/33/13639
- https://agupubs.onlinelibrary.wiley.com/doi/full/10.1029/2011JD016649
- https://www.britannica.com/science/chlorine/Physical-and-chemical-properties
- https://pubchem.ncbi.nlm.nih.gov/compound/chlorine#section=Solubility
- https://arc.aiaa.org/doi/abs/10.2514/3.4941?journalCode=aiaaj
- http://www.essentialchemicalindustry.org/chemicals/titanium-dioxide.html
- https://chlorine.americanchemistry.com/Chlorine/Chlorine-Benefits/Energy-and-Environment/
- https://sciencing.com/effects-chlorine-water-conductivity-6661358.html
- https://www.galaxywire.com/custom-wire-cable/jacket-insulation/pvc-polyvinyl-chloride/
- https://education.seattlepi.com/chlorine-gas-negative-effect-environment-6120.html
- http://www.chlorinethings.eu/home/blog/improving-stuff-with-chlorine-chemistry/transportation-solutions/chlorine-use-helps-protecting-marine-ecosystems
- https://www.cdc.gov/healthywater/drinking/public/chlorine-disinfection.html
- https://chlorine.americanchemistry.com/Chlorine-Benefits/Economic-Benefits/Crop-Protection-Compounds.pdf
- https://www.ncbi.nlm.nih.gov/pubmed/15092455
- http://www.rsc.org/periodic-table/element/17/chlorine
- https://www.lenntech.com/periodic/elements/cl.htm
- https://chlorine.americanchemistry.com/Chlorine-Benefits/Products-of-the-Chlorine-Tree.pdf
- http://iopscience.iop.org/article/10.1086/305343/meta
Cite This Work
To export a reference to this article please select a referencing style below:


