Research Booklet
The occasional table is an unthinkable game plan of the compound components, requested by their nuclear (number of protons), electron setups, and repeating concoction properties. This requesting indicates intermittent patterns, for example, components with comparative conduct in a similar segment.
It additionally indicates four rectangular pieces with some roughly comparable compound properties.
When all is said in done, inside one line (period) the components are metals on the left, and non-metals on the privilege.
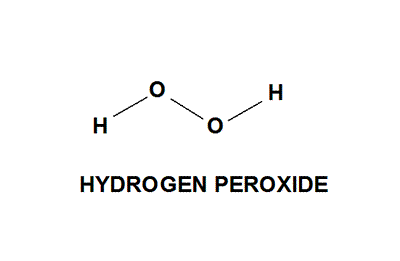
Hydrogen peroxide (H2O2) is an exceptionally light blue fluid which seems boring in a weaken arrangement, somewhat more thick than water.
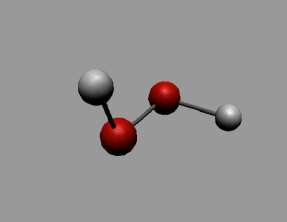 It is a powerless corrosive. It has solid oxidizing properties and is in this manner a capable fading operator that is generally utilized for fading paper, yet has additionally discovered use as a disinfectant and as an oxidizer.
It is a powerless corrosive. It has solid oxidizing properties and is in this manner a capable fading operator that is generally utilized for fading paper, yet has additionally discovered use as a disinfectant and as an oxidizer.
Hydrogen peroxide as carbamide peroxide is generally utilized for tooth brightening (blanching), both in professionally-and in self-managed items. Hydrogen peroxide (H2O2) is a very much recorded segment of living cells.
It assumes essential parts in host resistance and oxidative biosynthetic responses. What’s more there is developing confirmation that at low levels, H2O2 additionally works as a flagging specialist, especially in higher life forms.
H2O2 has progressively been an essential cell flagging operator in its own particular right, fit for tweaking both contractile and development advancing pathways with additional sweeping impacts.
Because of the gathering of hydrogen peroxide in the skin of patients with the depigmentation issue vitiligo, the human epidermis can’t have the ordinary limit with regards to autocrine union, transport and debasement of acetylcholine and the muscarinic (m1-m5) and nicotinic flag transduction in keratinocytes and melanocytes.
Gathering proof proposes that hydrogen peroxide (H(2)O(2)) assumes a critical part in growth advancement.
Trial information have demonstrated that growth cells deliver high measures of H(2)O(2).
The rate of a response can be expanded by including a reasonable impetus. An impetus is a substance which changes the rate of response yet is unaltered toward the finish of the response.
Just a little measure of impetus is expected to build the rate of response between a lot of reactants.
An impetus is to a specific response:
– Different impetuses catalyse diverse responses
– Not all responses have reasonable impetuses
The table outlines some basic impetuses utilized as a part of industry and the responses they catalyse.
The impact hypothesis clarifies that gas-stage substance responses happen when particles slam into adequate active vitality. The impact hypothesis depends on the Kinetic hypothesis of gasses; accordingly, just managing gas-stage compound responses are managed. Perfect gas suspicions are connected. Moreover, we additionally are expecting:
- All atoms are going through space in a straight line.
- All atoms are unbending circles.
- The responses concerned are between just two atoms.
- The atoms need to impact.
Eventually, the impact hypothesis of gasses gives the rate steady for bimolecular gas-stage responses; it is equivalent to the rate of effective crashes. The rate of effective impacts is corresponding to the part of fruitful crashes duplicated by the general impact recurrence.
Substance energy is the investigation of the rates of synthetic responses or how quick responses happen.
The essential prerequisite for a response to happen is that the reactant particles (iotas or atoms) must impact and communicate with each other somehow.
This is the focal thought of the crash show, which is utilized to clarify many the perceptions made about synthetic energy.
Crash hypothesis expresses that the rate of a concoction response is corresponding to the quantity of impacts between reactant atoms. The more regularly reactant atoms impact, the all the more frequently they respond with each other, quicker the response rate. In all actuality, just a little portion of the crashes are powerful impacts. Compelling impacts are those that outcome in a compound response.
Keeping in mind the end goal to create a compelling crash, reactant particles must have some base measure of vitality.
This vitality, used to start the response, is known as the initiation vitality. For each specimen of reactant particles there will be some that have this measure of vitality. The bigger the specimen, the more prominent the quantity of powerful impacts, and the speedier the rate of response. The quantity of particles having enough vitality is subject to the temperature of the reactants.
On the off chance that reactant particles don’t have the required enactment vitality when they impact, they bob off each other without responding.
Some substance responses likewise require that the reactant particles be in a specific introduction to deliver a viable crash.
Unless the reactant particles have this introduction when they impact, the crash won’t be a compelling one.
The response of ozone with nitrogen monoxide is a case of how introduction can be imperative.
In 1913, Neils Bohr, an understudy of Rutherford’s, produced another model of the iota. He suggested that electrons are masterminded in concentric roundabout circles around the core. This model is designed on the nearby planetary group and is known as the planetary model. The Bohr model can be compressed by the accompanying four standards:
- Electrons possess just certain circles around the core. Those circles are steady and are called “stationary” circles.
- Each circle has a vitality related with it. The circle closest the core has a vitality of E1, the following circle E2, and so forth.
- Vitality is consumed when an electron bounced from a lower circle to a higher one and vitality is radiated when an electron tumbles from a higher circle to a lower circle.
- The vitality and recurrence of light radiated or consumed can be figured by utilizing the distinction between the two orbital energies.
In 1926 Erwin Schrödinger, an Austrian physicist, took the Bohr molecule display above and beyond. Schrödinger utilized scientific conditions to portray the probability of finding an electron in a specific position. This nuclear model is known as the quantum mechanical model of the molecule. Not at all like the Bohr display, the quantum mechanical model does not characterize the correct way of an electron, but instead, predicts the chances of the area of the electron. This model can be depicted as a core encompassed by an electron cloud. Where the cloud is most thick, the likelihood of finding the electron is most noteworthy, and then again, the electron is more averse to be in a less thick territory of the cloud. Hence, this model presented the idea of sub-vitality levels.
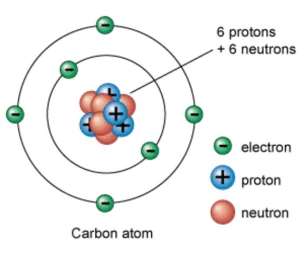
Until 1932, the molecule was accepted to be made out of a decidedly charged core encompassed by contrarily charged electrons. In 1932, James Chadwick shelled beryllium iotas with alpha particles. An obscure radiation was delivered. Chadwick translated this radiation as being made out of particles with an impartial electrical charge and the rough mass of a proton. This molecule got to be distinctly known as the neutron. With the revelation of the neutron, a satisfactory model of the iota got to be distinctly accessible to scientists.
Since 1932, through proceeded with experimentation, numerous extra particles have been found in the molecule. Likewise, new components have been made by besieging existing cores with different subatomic particles. The nuclear hypothesis has been further upgraded by the idea that protons and neutrons are made of much littler units called quarks. The quarks themselves are thus made of vibrating strings of vitality. The hypothesis of the creation of the iota keeps on being a continuous and energizing experience.
Science tubes are accessible in a large number of lengths and widths, normally from 10 to 20 mm wide and 50 to 200 mm long. The top regularly includes a flared lip to help spilling out the substance.
A science test tube normally has a level base, a round base, or a funnel shaped base. Some test tubes are made to acknowledge a ground glass plug or a screw top. They are frequently furnished with a little ground glass or white coating range close to the top for naming with a pencil.
Cite This Work
To export a reference to this article please select a referencing style below: