Ryan Lewis
Discuss current ideas about the origins of tissue macrophages and whether these origins influence the subsequent functions of macrophage
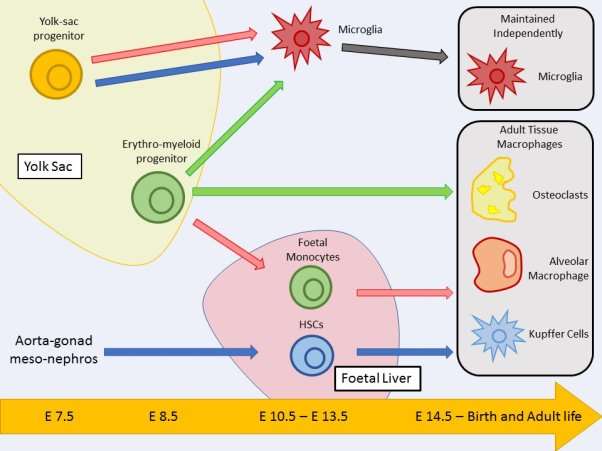
Graphical Abstract
Figure Legend: This figure highlights possible tissue macrophage origins and their development pathways. The essay discusses contradicting findings in the literature, involving three different publications; Sheng et al, 2015 (blue pathway), Hoeffel et al, 2015 (red pathway) and Perdiguero et al, 2015 (green pathway) which are shown in the figure. Cell positions relate to the time point they are established during embryonic development. Positions of yolk sac and foetal liver are also related to the times they are developed.
Introduction
In the late 19th century Ilya Metchnikoff discovered macrophages (Tauber, 2003) and since then our understanding of the immune system and its complexity has progressed to a stage where the macrophage is no longer as simple as was originally depicted by Metchnikoff. Although much more is known about tissue specific macrophages and their functions, the origins of these macrophages are less well understood including how their origins relate to the functions they have within specific tissues. This essay aims to address the current ideas about the origins of tissue macrophages and whether these origins influence the subsequent functions of macrophages.
Macrophage Discovery and History
As previously mentioned Metchnikoff discovered the macrophage late in the 19th century (Tauber, 2003). Metchnikoff published a paper talking about phagocytic cells he had observed in frogs, he described the phagocytic cells as being involved in host defence but also the clearing of dead and dying cells (Gordon, 2007). Mechnikoff then discovered the presence macrophages in starfish, which don’t have a vascular system, which led him to the discovery of tissue-resident macrophages (Gordon, 2007). Metchnikoff received the Nobel prize for his studies on cellular immunity to infection in vertebrates which he shared with Paul Ehrlich who discovered humoral immunity (Gordon, 2007). It took roughly 80 years after Metchnikoff’s discovery before the origin of the tissue macrophage was uncovered. It was proposed that tissue macrophages originated from circulating monocytes in the blood (van Furth and Cohn, 1968), this theory has persisted for the last 40 years however from recent studies we know that this is not the primary origin of the tissue macrophage. Shortly after the theory that tissue macrophages originated from circulating monocytes was proposed, it was discovered that tissue macrophages and monocytes are heterogenous and their heterogeneity is conserved in humans and mice (Gordon and Taylor, 2005). The discovery of monocyte subsets followed shortly after in 1983, which supported the theory that tissue macrophages originated from circulating monocytes (Yona and Jung, 2009). The theory that tissue macrophages are derived from circulating monocytes has been the prevailing view until very recently partly due to the arrival of advanced techniques including; fate mapping and ionizing radiation. In the last 5-6 years, many definitive publications have redefined our understanding of the origins of tissue macrophages (Epelman et al, 2014). Recent studies have shown that many tissue macrophages are established during embryonic development and continually self-replenish into adulthood independently of any input from circulating monocytes in the blood (Epelman et al, 2014; Ginhoux et al, 2010; Hashimoto et al, 2013; Yona et al, 2013).
Tissue Macrophage Heterogeneity and Function
Tissue macrophage have a huge degree of heterogeneity which reflects upon the specialization of their functions in different tissues and locations (Gordon and Taylor, 2005). Macrophage heterogeneity is required to ensure the tissue macrophage has the most effective phenotype to tackle its specific microenvironment, this is particularly important in the gut. Tissue macrophages in the gut isolated from the lamina propria have a unique phenotype characterised by high phagocytic and bactericidal activity but very poor production of pro-inflammatory cytokines which makes them perfectly suited to their microenvironment (Gordon and Taylor, 2005). There are many specialised tissue macrophages that have very distinct functions including; osteoclasts in the bone which breakdown bone deposits for bone remodelling, alveolar macrophages (dust cells) in the lung that break down foreign material and pathogens, and microglia in the brain which play a role in neuronal development homeostasis and the recovery from pathology (Boyce et al, 2008; Rubins, 2003; Prinz et al, 2014). The theory that tissue macrophage populations are replenished from circulating monocytes in the blood is somewhat true but the most diverse tissue macrophages such as microglia, alveolar macrophages and osteoclasts are replenished through self-renewal and proliferation (Yona and Jung, 2009). There is a substantial number of studies discussing whether macrophages originating from monocytes in the blood can differentiate into resident tissue macrophages. In most cases the monocyte subset that the macrophage originated from determines its ability to differentiate into a specialized resident tissue macrophage, this is particularly true in the lung as studies have shown only Ly6Clo, not Ly6Chi, monocytes have the ability to differentiate into enchymal lung macrophages (Landsman et al, 2007). In regards to the more complex and specialised alveolar macrophages in the lung, studies have shown that these macrophages require a parenchymal lung macrophage intermediate (Landsman and Jung, 2007). Circulating monocytes in the blood were long believed to be the origin of specialised tissue macrophages but recent evidence has shown that this is incorrect and proven that many of these tissue macrophage populations are developed long before birth (Epelman et al, 2014).
Origins of Tissue Macrophages
Macrophages are first observed during embryonic day 6.5 and are produced in the yolk sac during what is termed as primitive haematopoiesis (Epelman et al, 2014). During this early stage in development macrophages are the only immune cell produced due to restricted progenitors in the yolk sac. During embryonic days 8.5 – 10.5 hematopoietic stem cells (HSCs) emerge from the aorta-gonad meso-nephros (AGM) and give rise to all immune lineages (Epelman et al, 2014). At embryonic day 10.5 HSCs migrate from the AGM to the foetal liver, the foetal liver then becomes the major hematopoietic organ until birth. Only after birth do bone marrow HSCs become the primary progenitors and produce all immune lineages (Orkin and Zon, 2008). Microglia are the only tissue macrophages that are established in the yolk sac and are self-maintained through-out adulthood, all the other tissue macrophages are established from embryonic day 14.5 to birth and either self-maintained by proliferation or replenished by HSCs in the bone marrow (Ginhoux et al, 2010; Sheng et al, 2015). The arrival of fat-mapping techniques have enabled researchers to precisely track embryonic macrophage populations into adulthood, giving an insight into the relationship between resident tissue macrophages and circulating blood monocytes (Epelman et al, 2014). As previously discussed, microglia are the only tissue macrophage originating from the yolk sac and arise before embryonic day 8 (Ginhoux et al 2010). Fate mapping analysis was used to determine that the origin of microglia was the primitive myeloid precursors in the yolk sac and also proved that microglia are self-maintained independently of any circulating blood monocytes (Ginhoux et al, 2010). There is also evidence that Langerhans cells originate from the yolk sac but only partially (Sheng et al, 2015). The fate mapping study by Sheng proved that microglia and Langerhans cells were the only tissue macrophages that originate from yolk sac precursors and that most adult tissue macrophages originate from a second wave of haematopoiesis driven by HSCs. (Sheng et al, 2015). The number recent of publications concerning tissue macrophage origins is staggering and is most likely attributed to the arrival of fate mapping techniques. With the large surge of new studies regarding tissue macrophage origins it is important that a clear understanding is generated but this is not always possible with such a complicated subject.
Contrasting Studies into Tissue Macrophage Origins
There are a few recent studies concerning tissue macrophage origins which are particularly interesting. Sheng (Sheng et al, 2015) arrived at the conclusion that most tissue macrophages originate from HSCs however there are a few publications which contradict Shengs findings. Perdiguero concluded that yolk sac derived erythro-myeloid progenitors, were origin of almost all tissue macrophages which contrasts greatly with Shengs observations. (Perdiguero et al, 2015). Perdiguero also concluded that microglia were derived from erythro-myeloid progenitors rather than primitive yolk sac progenitors that was observed by Sheng, although both do come from the yolk sac (Perdiguero et al, 2015; Sheng et al, 2015). Perdiguero predicted that almost all other tissue macrophages originated from erythro-myeloid progenitors (Perdiguero et al, 2015; Sheng et al, 2015). A study by Hoeffel aligned well with Perdigueros observations but Hoeffel observed that primitive yolk sac progenitors gave rise to microglia rather than erythro-myeloid progenitors that was observed by Perdiguero (Hoeffel et al, 2015; Perdiguero et al, 2015). As well as the difference in the development of microglia, Hoeffel predicted that erythro-myeloid progenitors migrated to the foetal liver, giving rise to foetal monocytes which were then responsible for the production of tissue macrophages. (Hoeffel et al, 2015). Each of these 3 examples also propose a separate proposed major path of ontogeny and differentiation to adult tissue macrophage state. Perdiguero proposes erythro-myeloid progenitors from the yolk sac as the major precursor of tissue macrophages, Heoffel proposes erythro-myeloid progenitors from the foetal liver, as foetal monocytes, as the major precursor and, Sheng proposes that HSCs from the foetal liver are the major precursor (Perdiguero et al, 2015; Hoeffel et al, 2015; Sheng et al, 2015; Guinhoux and Guilliams, 2016). Although the observations made by Sheng are profoundly different to those made by Perdiguero and Hoeffel it could be down to the fate mapping technique they used. The model they used is not adapted to distinguish between late erythro-myeloid progenitors and foetal HSCs which has clearly effected the conclusion they have come to (Guinhoux and Guilliams, 2016). Although fate mapping has great potential in advancing our knowledge of cellular ontogeny there are certain limitation that come with it and these limitations must be considered when designing experiments and analysing data (Guinhoux and Guilliams, 2016).
Do Tissue Macrophage Origins Matter?
Determining the origins of tissue macrophages may be valuable for furthering our knowledge and understanding of their development but do their origins have any influence in determining their function? As well as ontogeny, diversity in the functions of tissue macrophages can also be attributed to the local signals received by the macrophages. These local changes can drive the expression of unique transcription factors which in turn lead to different functions (Lavin et al, 2015). There is a lot of evidence to suggest that the tissue macrophages microenvironment can alter its function, the plasticity of tissue macrophages allows them to adjust their functions to inflammatory events (Lavin et al, 2015). Using ionizing radiation most embryonic-derived tissue macrophages can be eliminated, they can then be replaced with donor-derived bone marrow progenitors to determine if the wild type state of the tissue can be restored. Using this technique, studies have proven that bone marrow progenitors can completely restore the enhancer profile and transcriptional programme of the embryonic-derived tissue macrophages that were eliminated (Lavin et al, 2015). A very recent study has shown that yolk sac macrophages, foetal liver monocytes and adult bone marrow monocytes can all successfully differentiate into alveolar macrophages in the lung after the removal of the native alveolar macrophages using ionizing radiation (van de Laar et al, 2016). The study also showed that other already developed tissue macrophages, liver, peritoneal and colon macrophages cannot successfully differentiate into alveolar macrophages in the lung. This finding suggests that the plasticity of the mononuclear phagocyte system is at its largest during the precursor stage and after differentiation to tissue-resident macrophages no further phenotypic changes of macrophage types can take place (van de Laar, 2016). Perhaps the most interesting finding from this study is that the alveolar macrophages differentiated from yolk sac macrophages, foetal liver monocytes and bone marrow monocytes were still able to self-maintain and prevent alveolar proteinosis (van de Laar, 2016). Similar results have also been observed with Kupffer cells. Kupffer cells were eliminated from the liver using diphtheria toxin-mediated depletion allowing its niche to become vacant. Observations showed that circulating monocytes can engraft the liver and adopt the transcriptional profile of the eliminated Kupffer cells and also become long-living self-renewing cells like their eliminated counterparts (Scott et al, 2015). These new findings question whether the origin of tissue macrophages is truly important to their function as the progenitors and monocytes tested have all been able to restore the tissues lost macrophages successfully without any loss of function.
Conclusion
Although determining the origins of tissue macrophages and other members of the immune system is important for the progression of our knowledge it remains to be seen whether the actual origins have any implications on the function of the tissue macrophages. The techniques used in the publications discussed are still very new and still require refinement, I believe further refinement of the techniques will enable a more detailed and accurate description on the origins of tissue macrophages and the role the origins play in their function.
References
BOYCE, B.F., YAO, Z. & XING, L. 2009
Osteoclasts have multiple roles in bone addition to bone resorption.
Critical Reviews in Eukaryotic Gene Expression, 19.3, 171-180
EPELMAN, S., LAVINE, K.J. & RANDOLPH, G.J. 2014
Origin and functions of tissue macrophages.
Immunity, 41.1, 21-35
GINHOUX, F., GRETER, M., LEBOEUF, M., NANDI, S., SEE, P., GOKHAN, S., MEHLER, M.F., CONWAY, S.J., GUAN NG, L., STANLEY, E.R., SAMOKHVALOV, I.M. & MERAD, M. 2010
Fate mapping analysis reveals that adult microglia derive from primitive macrophages.
Science, 330.6005, 841-845
GUINHOUX, F. & GUILLIAMS 2016
Tissue-resident macrophage ontogeny and homeostasis.
Immunity, 44.3, 439-449
GORDON, S. 2007
The macrophage: past, present and future.
European Journal of Immunology, 37, 9-17
GORDON, S. & TAYLOR, P.R. 2005
Monocyte and macrophage heterogeneity.
Nature Reviews: Immunology, 5.12, 953-964
HASHIMOTO, D., CHOW, A., NOIZAT, C., TEO, P., BEASLEY, M.B., LEBOEUF, M., BECKER, C.D., SEE, P., PRICE, J., LUCAS, D., GRETER, M., MORTHA, A., BOYER, S.W., FORSBERG, E.C., TANAKA, M., VAN ROOIJEN, N., GARCIA-SASTRE, A., STANLEY, E.R., GINHOUX, F., FRENETTE, P.S. & MERAD, M. 2013
Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes.
Immunity, 38.4, 792-804
HOEFFEL, G., CHEN, J., LAVIN, Y., LOW, D., ALMEIDA, F.F., SEE, P., BEAUDIN, A.E., LUM, J., LOW, I., FORSBERG, E.C, POIDINGER, M., ZOLEZZI, F., LARBI, A., NG, L.G., CHAN, J.K., GRETER, J.K., BECHER, B., SAMOKHVALOV, I.M., MERAD, M. & GINHOUX, F. 2015
C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages.
Immunity, 42.4, 665-678
LANDSMAN, L., VAROL, C. & JUNG, S. 2007
Distinct differentiation potential of blood monocyte subsets in the lung.
Journal of Immunology, 178.4, 2000-2007
LANDSMAN, L. & JUNG, S. 2007
Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages.
Journal of Immunology, 179.6, 3488-3494
LAVIN, Y., MORTHA, A., RAHMAN, A. & MERAD, M. 2016
Regulation of macrophage development and function in peripheral tissues.
Nature Reviews: Immunology, 15.12, 731-744
ORKIN, S.H. & ZON, L.I. 2008
Haematopoiesis: an evolving paradigm for stem cell biology.
Cell, 132, 631-644
PERDIGUERO, E.G., KLAPPROTH, K., SCHULZ, C., BUSCH, K., AZZONI, E., CROZET, L., GARNER, H., TROUILLET, C., DE BRUIJN, M.F., GEISSMANN, F. & RODEWALD, H.R. 2014
Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors.
Nature, 518, 547-551
PRINZ, M., TAY, T.L., WOLF, Y. & JUNG, S. 2014
Microglia: unique and common features with other tissue macrophages.
Acta Neuropathologica, 128.3, 319-331
RUBINS, J.B. 2003
Alveolar macrophages: wielding the double-edged sword of inflammation.
American Journal of Respiratory and Critical Care Medicine, 167.2, 103-104
SCOTT, C.L., ZHENG, F., DE BAETSELIER, P., MARTENS, L., SAEYS, Y., DE PRIJCK, S., LIPPENS, S., ABELS, C., SCHOONOOGHE, S., RAES, G., DEVOOGDT, N., LAMBRECHT, B.N., BESCHIN, A. & GUILLIAMS, M. 2016
Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells.
Nature Communications, 7, 10321
SHENG, J., RUEDL, C. & KARJALAINEN, K. 2015
Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells.
Immunity, 43.2, 382-393
TAUBER, A.I. 2003
Metchnikoff and the phagocytosis theory.
Nature Reviews: Molecular Cell Biology, 4, 897-901
VAN DE LAAR, L., SAELENS, W., DE PRIJCK, S., MARTENS, L., SCOTT, C.L., VAN ISTERDAEL, G., HOFFMANN, E., BEYAERT, R., SAEYS, Y., LAMBRECHT, B.N. & GUILLIAMS, M. 2016
Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages.
Immunity, 44.4, 755-768
VAN FURTH, R. & COHN, Z.A. 1968
The origin and kinetics of mononuclear phagocytes.
The Journal of Experimental Medicine, 128.3, 415-435
YONA, S., KIM, K.W., WOLF, Y., MILDNER, A., VAROL, D., BRECKER, M., STRAUSS-AYALI, D., VIUKOV, S., GUILLIAMS, M., MISHARIN, A., HUME, D.A., PERLMAN, H., MALISSEN, B., ZELZER, E. & JUNG, S. 2013
Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis.
Immunity, 38.1, 79-91
YONA, S. & JUNG, S. 2009
Monocytes: subsets, origins, fates, and functions.
Current Opinion in Hematology, 17.1, 53-59
Cite This Work
To export a reference to this article please select a referencing style below:


